Research Paper and Review
2024
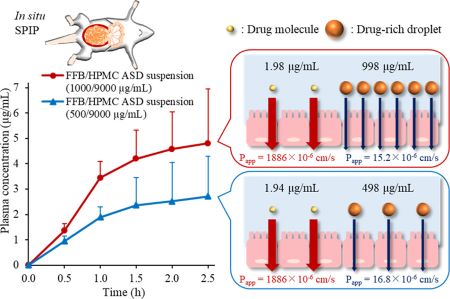 Yoshikawa, E., Ueda, K., Hakata, R., Higashi, K., Moribe, K.: Quantitative investigation of intestinal drug absorption enhancement by drug-rich nanodroplets generated via liquid–liquid phase separation. Mol. Pharm., 21 (4), 1745-1755 (2024). https://doi.org/10.1021/acs.molpharmaceut.3c01078
Yoshikawa, E., Ueda, K., Hakata, R., Higashi, K., Moribe, K.: Quantitative investigation of intestinal drug absorption enhancement by drug-rich nanodroplets generated via liquid–liquid phase separation. Mol. Pharm., 21 (4), 1745-1755 (2024). https://doi.org/10.1021/acs.molpharmaceut.3c01078
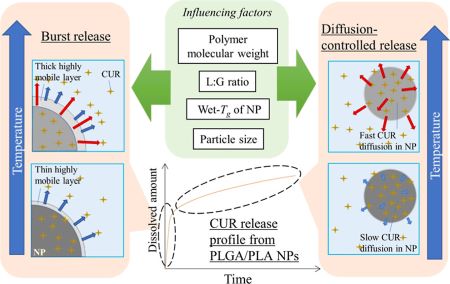 Sunazuka, Y., Ueda, K., Higashi, K., Wada, K., Moribe, K.: Mechanistic analysis of temperature-dependent curcumin release from poly(lactic-co-glycolic acid)/poly(lactic acid) polymer nanoparticles. Mol. Pharm., 21(3), 1424-1435 (2024). https://doi.org/10.1021/acs.molpharmaceut.3c01066
Sunazuka, Y., Ueda, K., Higashi, K., Wada, K., Moribe, K.: Mechanistic analysis of temperature-dependent curcumin release from poly(lactic-co-glycolic acid)/poly(lactic acid) polymer nanoparticles. Mol. Pharm., 21(3), 1424-1435 (2024). https://doi.org/10.1021/acs.molpharmaceut.3c01066
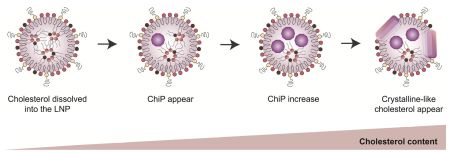 Anindita, J., Tanaka, H., Yamakawa, T., Sato, Y., Matsumoto, C., Ishizaki, K., Oyama, T., Suzuki, S., Ueda, K., Higashi, K., Moribe, K., Sasaki, K., Ogura, Y., Yonemochi, E., Sakurai, Y., Hatakeyama, H., Akita, H.: The Effect of Cholesterol Content on the Adjuvant Activity of Nucleic-Acid-Free Lipid Nanoparticles. Pharmacuetics, 16(2), 181 (2024). https://doi.org/10.3390/pharmaceutics16020181
Anindita, J., Tanaka, H., Yamakawa, T., Sato, Y., Matsumoto, C., Ishizaki, K., Oyama, T., Suzuki, S., Ueda, K., Higashi, K., Moribe, K., Sasaki, K., Ogura, Y., Yonemochi, E., Sakurai, Y., Hatakeyama, H., Akita, H.: The Effect of Cholesterol Content on the Adjuvant Activity of Nucleic-Acid-Free Lipid Nanoparticles. Pharmacuetics, 16(2), 181 (2024). https://doi.org/10.3390/pharmaceutics16020181
2023
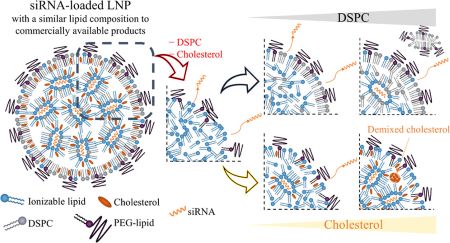 Ueda, K., Sakagawa, Y., Saito, T., Fujimoto, T., Nakamura, M., Sakuma, F., Kaneko, S., Tokumoto, T., Nishimura, K., Takeda, J., Arai, Y., Yamamoto, K., Ikeda, Y., Higashi, K., Moribe, K.: Molecular-level structural analysis of siRNA-loaded lipid nanoparticles by 1H NMR relaxometry: Impact of lipid composition on their structural properties. Mol. Pharm., 20(9), 4729-4742 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00477
Ueda, K., Sakagawa, Y., Saito, T., Fujimoto, T., Nakamura, M., Sakuma, F., Kaneko, S., Tokumoto, T., Nishimura, K., Takeda, J., Arai, Y., Yamamoto, K., Ikeda, Y., Higashi, K., Moribe, K.: Molecular-level structural analysis of siRNA-loaded lipid nanoparticles by 1H NMR relaxometry: Impact of lipid composition on their structural properties. Mol. Pharm., 20(9), 4729-4742 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00477
Miyazaki, T., Mizoguchi, R., Ueda, K., Shinozaki, T., Kamoto, M., Takeda, Y., Sakuma, S., Ito, N., Momo, M., Kawakami, K.: Crystallization of amorphous nifedipine under isothermal conditions: Inter-laboratory reproducibility and investigation of the factors affecting reproducibility. J. Pharm. Sci., 112(10), 2703-2716 (2023). https://doi.org/10.1016/j.xphs.2023.06.002
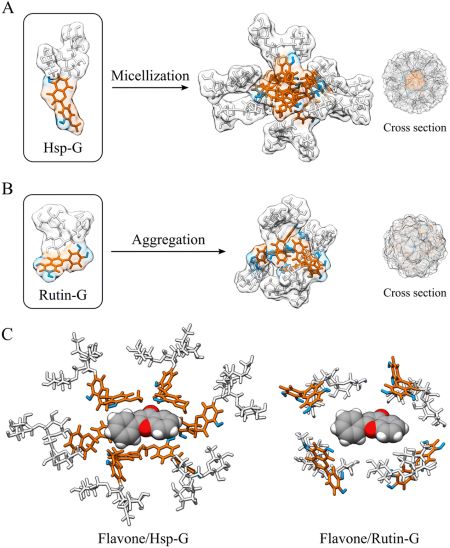 Kadota, K., Kämäräinen, T., Sakuma, F., Ueda, K., Higashi, K., Moribe, K., Uchiyama, H., Minoura, K., Tozuka, Y.: Unveiling the flavone-solubilizing effects of α-glucosyl rutin and hesperidin: probing structural differences through NMR and SAXS analyses. Food. Funct., 14, 10493-10505 (2023). https://doi.org/10.1039/D3FO03261B
Kadota, K., Kämäräinen, T., Sakuma, F., Ueda, K., Higashi, K., Moribe, K., Uchiyama, H., Minoura, K., Tozuka, Y.: Unveiling the flavone-solubilizing effects of α-glucosyl rutin and hesperidin: probing structural differences through NMR and SAXS analyses. Food. Funct., 14, 10493-10505 (2023). https://doi.org/10.1039/D3FO03261B
 Hanada, N., Higashi, K., Zhao, Z., Ueda, K., Moribe, K.: Preparation of a ternary amorphous solid dispersion using hot-melt extrusion for obtaining a stable colloidal dispersion of amorphous probucol nanoparticles. Int. J. Pharm., 640, 122959 (2023). https://doi.org/10.1016/j.ijpharm.2023.122959
Hanada, N., Higashi, K., Zhao, Z., Ueda, K., Moribe, K.: Preparation of a ternary amorphous solid dispersion using hot-melt extrusion for obtaining a stable colloidal dispersion of amorphous probucol nanoparticles. Int. J. Pharm., 640, 122959 (2023). https://doi.org/10.1016/j.ijpharm.2023.122959
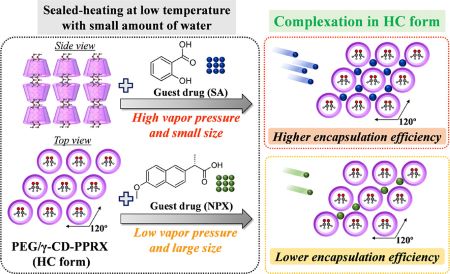 Kundu, S., Higashi, K., Takamizawa, M., Ueda, K., Moribe, K.: Vapor-phase-mediated encapsulation of guest drug molecules in the hexagonal columnar form structure of polyethylene glycol/γ-cyclodextrin-polypseudorotaxane. Cryst. Growth. Des., 23(8), 6119-6129 (2023). https://doi.org/10.1021/acs.cgd.3c00619
Kundu, S., Higashi, K., Takamizawa, M., Ueda, K., Moribe, K.: Vapor-phase-mediated encapsulation of guest drug molecules in the hexagonal columnar form structure of polyethylene glycol/γ-cyclodextrin-polypseudorotaxane. Cryst. Growth. Des., 23(8), 6119-6129 (2023). https://doi.org/10.1021/acs.cgd.3c00619
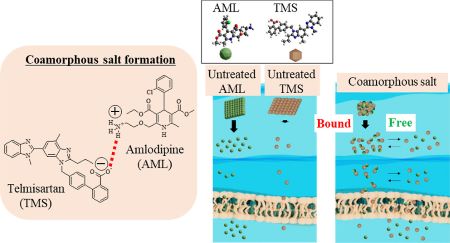 Hatanaka, Y., Uchiyama, H., Kaneko, S., Ueda, K., Higashi, K., Moribe, K., Furukawa, S., Takase, M., Yamanaka, S., Kadota, K., Tozuka, Y.: Designing a novel coamorphous salt formulation of telmisartan with amlodipine to enhance permeability and oral absorption. Mol. Pharm., 20(8), 4071-4085 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00226
Hatanaka, Y., Uchiyama, H., Kaneko, S., Ueda, K., Higashi, K., Moribe, K., Furukawa, S., Takase, M., Yamanaka, S., Kadota, K., Tozuka, Y.: Designing a novel coamorphous salt formulation of telmisartan with amlodipine to enhance permeability and oral absorption. Mol. Pharm., 20(8), 4071-4085 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00226
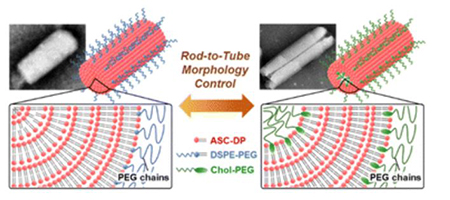 Chen, Z., Higashi, K., Shigehisa, Y., Ueda, K., Yamamoto, K., Moribe, K.: Understanding the rod-to-tube transformation of self-assembled ascorbyl dipalmitate lipid nanoparticles stabilized with PEGylated lipids. Nanoscale, 15, 2602-2613 (2023). https://doi.org/10.1039/D2NR04987B
Chen, Z., Higashi, K., Shigehisa, Y., Ueda, K., Yamamoto, K., Moribe, K.: Understanding the rod-to-tube transformation of self-assembled ascorbyl dipalmitate lipid nanoparticles stabilized with PEGylated lipids. Nanoscale, 15, 2602-2613 (2023). https://doi.org/10.1039/D2NR04987B
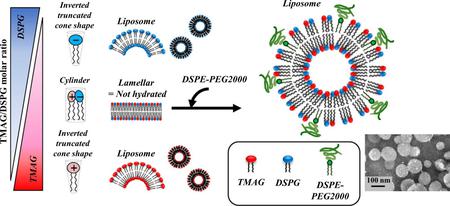 Hohokabe, M., Higashi, K., Yamada, Y., Fujimoto, T., Tokumoto, T., Imamura, H., Morita, T., Ueda, K., Limwikrant, W., Moribe, K.: Modification of liposomes composed of a cationic lipid TMAG and an anionic lipid DSPG with a PEGylated lipid based on the investigation of lipid structures. Colloid. Surf. A: Phys. Eng. Asp., 661, 130981 (2023). https://doi.org/10.1016/j.colsurfa.2022.130891
Hohokabe, M., Higashi, K., Yamada, Y., Fujimoto, T., Tokumoto, T., Imamura, H., Morita, T., Ueda, K., Limwikrant, W., Moribe, K.: Modification of liposomes composed of a cationic lipid TMAG and an anionic lipid DSPG with a PEGylated lipid based on the investigation of lipid structures. Colloid. Surf. A: Phys. Eng. Asp., 661, 130981 (2023). https://doi.org/10.1016/j.colsurfa.2022.130891
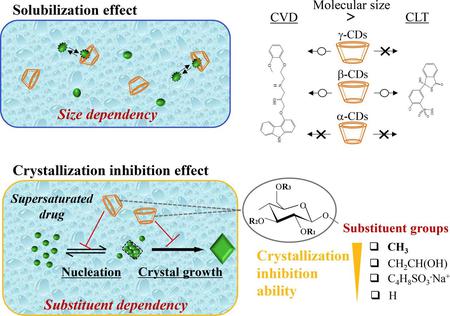 Liu, M., Higashi, K., Ueda, K., Moribe, K.: Supersaturation maintenance of carvedilol and chlorthalidone by cyclodextrin derivatives: Pronounced crystallization inhibition ability of methylated cyclodextrin. Int. J. Pharm., 637, 122876 (2023). https://doi.org/10.1016/j.ijpharm.2023.122876
Liu, M., Higashi, K., Ueda, K., Moribe, K.: Supersaturation maintenance of carvedilol and chlorthalidone by cyclodextrin derivatives: Pronounced crystallization inhibition ability of methylated cyclodextrin. Int. J. Pharm., 637, 122876 (2023). https://doi.org/10.1016/j.ijpharm.2023.122876
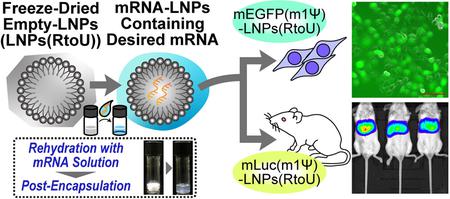 Tanaka, H., Hagiwara, S., Shirane, D., Yamakawa, T., Sato, Y., Matsumoto, C., Ishizaki, K., Hishinuma, M., Chida, K., Sasaki, K., Yonemochi, E., Ueda, K., Higashi, K., Moribe, K., Tadokoro, T., Maenaka, K., Taneichi, S., Nakai, Y., Tange, K., Sakurai, Y., Akita, H.: Ready-to-use-type lyophilized lipid nanoparticle formulation for the postencapsulation of messenger RNA. ACS Nano, 17(3), 2588-2601 (2023). https://doi.org/10.1021/acsnano.2c10501
Tanaka, H., Hagiwara, S., Shirane, D., Yamakawa, T., Sato, Y., Matsumoto, C., Ishizaki, K., Hishinuma, M., Chida, K., Sasaki, K., Yonemochi, E., Ueda, K., Higashi, K., Moribe, K., Tadokoro, T., Maenaka, K., Taneichi, S., Nakai, Y., Tange, K., Sakurai, Y., Akita, H.: Ready-to-use-type lyophilized lipid nanoparticle formulation for the postencapsulation of messenger RNA. ACS Nano, 17(3), 2588-2601 (2023). https://doi.org/10.1021/acsnano.2c10501
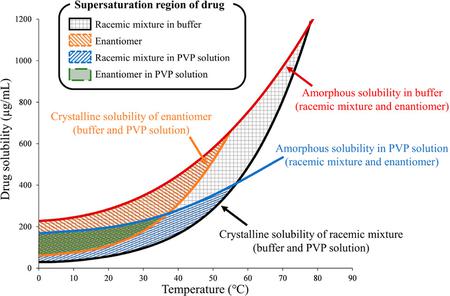 Ueda, K., Higashi, K., Moribe, K.: Quantitative analysis of drug supersaturation region by temperature-variable nuclear magnetic resonance measurements, part 1: Effects of polymer and drug chiralities. Mol. Pharm., 20(4), 1861-1871 (2023). https://doi.org/10.1021/acs.molpharmaceut.2c00924
Ueda, K., Higashi, K., Moribe, K.: Quantitative analysis of drug supersaturation region by temperature-variable nuclear magnetic resonance measurements, part 1: Effects of polymer and drug chiralities. Mol. Pharm., 20(4), 1861-1871 (2023). https://doi.org/10.1021/acs.molpharmaceut.2c00924
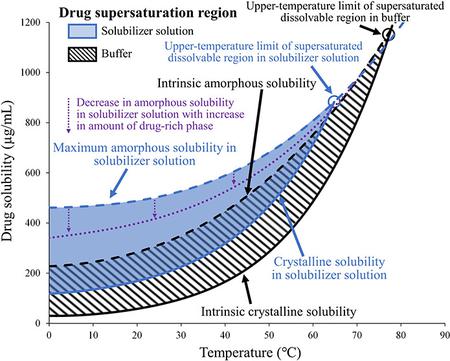 Ueda, K., Higashi, K., Moribe, K.: Quantitative analysis of drug supersaturation region by temperature-variable nuclear magnetic resonance measurements, part 2: Effects of solubilizer. Mol. Pharm., 20(4), 1872-1883 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00050
Ueda, K., Higashi, K., Moribe, K.: Quantitative analysis of drug supersaturation region by temperature-variable nuclear magnetic resonance measurements, part 2: Effects of solubilizer. Mol. Pharm., 20(4), 1872-1883 (2023). https://doi.org/10.1021/acs.molpharmaceut.3c00050
2022
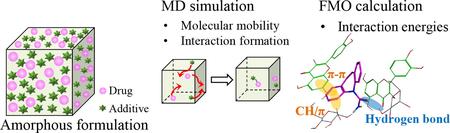 Ma, X., Higashi, K., Fukuzawa, K., Ueda, K., Kadota, K., Tozuka, Y., Yonemochi, E., Moribe, K.: Computational approach to elucidate the formation and stabilization mechanism of amorphous formulation using molecular dynamics simulation and fragment molecular orbital calculation. Int. J. Pharm., 615(5), 121477 (2022). https://doi.org/10.1016/j.ijpharm.2022.121477
Ma, X., Higashi, K., Fukuzawa, K., Ueda, K., Kadota, K., Tozuka, Y., Yonemochi, E., Moribe, K.: Computational approach to elucidate the formation and stabilization mechanism of amorphous formulation using molecular dynamics simulation and fragment molecular orbital calculation. Int. J. Pharm., 615(5), 121477 (2022). https://doi.org/10.1016/j.ijpharm.2022.121477
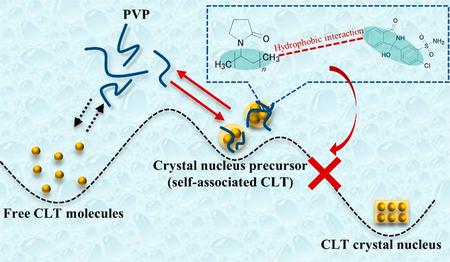 Ueda, K., Yamamoto, N., Higashi, K., Moribe, K.: NMR-based mechanistic study of crystal nucleation inhibition in a supersaturated drug solution by polyvinylpyrrolidone. Cryst. Growth. Des., 22(5), 3535-3544 (2022). https://doi.org/10.1021/acs.cgd.2c00084
Ueda, K., Yamamoto, N., Higashi, K., Moribe, K.: NMR-based mechanistic study of crystal nucleation inhibition in a supersaturated drug solution by polyvinylpyrrolidone. Cryst. Growth. Des., 22(5), 3535-3544 (2022). https://doi.org/10.1021/acs.cgd.2c00084
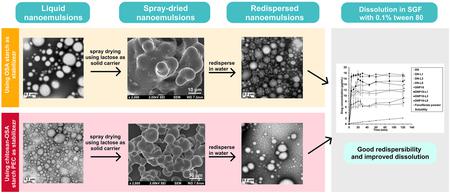 Sodalee, K., Limwikrant, W., Pongjanyakul, T., Ueda, K., Higashi, K., Moribe, K., Puttipipatkhachorn, S.: Preparation of redispersible dry nanoemulsion using chitosan-octenyl succinic anhydride starch polyelectrolyte complex as stabilizer. J. Drug. Deliv. Sci. Technol., 73, 103433 (2022). https://doi.org/10.1016/j.jddst.2022.103433
Sodalee, K., Limwikrant, W., Pongjanyakul, T., Ueda, K., Higashi, K., Moribe, K., Puttipipatkhachorn, S.: Preparation of redispersible dry nanoemulsion using chitosan-octenyl succinic anhydride starch polyelectrolyte complex as stabilizer. J. Drug. Deliv. Sci. Technol., 73, 103433 (2022). https://doi.org/10.1016/j.jddst.2022.103433
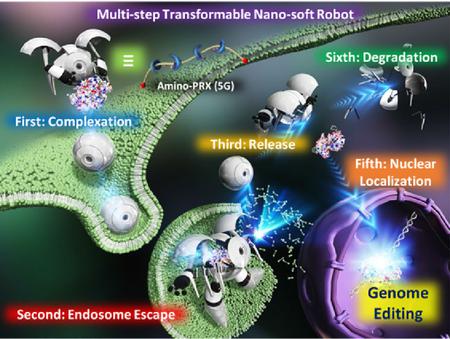 Taharabaru, T., Kihara, T., Onodera, R., Kogo, T., Higashi, K., Moribe, K., Nakamura, T., Motoyama, K., Higashi, T.: Polyrotaxane-based multi-step transformable materials for the delivery of Cas9 ribonucleoprotein. Appl. Mater. Today, 27, 101488 (2022). https://doi.org/10.1016/j.apmt.2022.101488
Taharabaru, T., Kihara, T., Onodera, R., Kogo, T., Higashi, K., Moribe, K., Nakamura, T., Motoyama, K., Higashi, T.: Polyrotaxane-based multi-step transformable materials for the delivery of Cas9 ribonucleoprotein. Appl. Mater. Today, 27, 101488 (2022). https://doi.org/10.1016/j.apmt.2022.101488
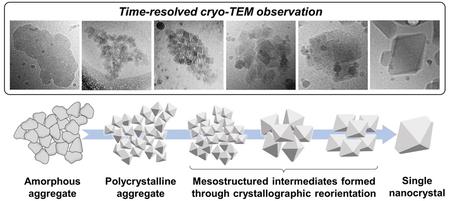 Chen, Z., Higashi, K., Ueda, K., Moribe, K.: Multistep crystallization of pharmaceutical amorphous nanoparticles via a cognate pathway of oriented attachment: Direct evidence of nonclassical crystallization for organic molecules. Nano. Lett., 22(16), 6841-6846 (2022). https://doi.org/10.1021/acs.nanolett.2c01608
Chen, Z., Higashi, K., Ueda, K., Moribe, K.: Multistep crystallization of pharmaceutical amorphous nanoparticles via a cognate pathway of oriented attachment: Direct evidence of nonclassical crystallization for organic molecules. Nano. Lett., 22(16), 6841-6846 (2022). https://doi.org/10.1021/acs.nanolett.2c01608
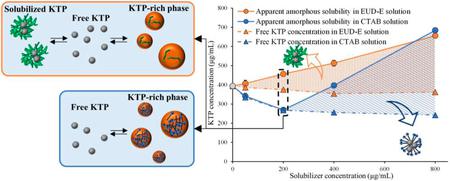 Ueda, K., Higashi, K., Moribe, K.: Unusual correlation between the apparent amorphous solubility of a drug and solubilizer concentration revealed by NMR analysis. Mol. Pharm., 19(9), 3336-3349 (2022). https://doi.org/10.1021/acs.molpharmaceut.2c00478
Ueda, K., Higashi, K., Moribe, K.: Unusual correlation between the apparent amorphous solubility of a drug and solubilizer concentration revealed by NMR analysis. Mol. Pharm., 19(9), 3336-3349 (2022). https://doi.org/10.1021/acs.molpharmaceut.2c00478
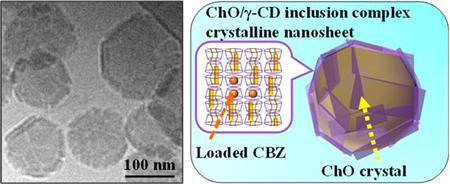 Ishimoto, A., Sasako, H., Omori, M., Higashi, K., Ueda, K., Koyama, K., Moribe, K.: Drug-loaded nanocarriers composed of cholesteryl oleate crystal cores and multiple-nanosheet shells of γ-cyclodextrin inclusion complex crystals. Langmuir, 38(34), 10454-10464 (2022). https://doi.org/10.1021/acs.langmuir.2c01199
Ishimoto, A., Sasako, H., Omori, M., Higashi, K., Ueda, K., Koyama, K., Moribe, K.: Drug-loaded nanocarriers composed of cholesteryl oleate crystal cores and multiple-nanosheet shells of γ-cyclodextrin inclusion complex crystals. Langmuir, 38(34), 10454-10464 (2022). https://doi.org/10.1021/acs.langmuir.2c01199
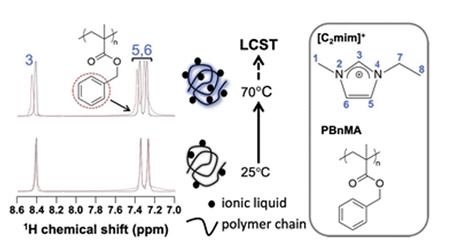 Morita, T., Okada, H., Yamada, T., Hidaka, R., Ueki, T., Niitsuma, K., Kitazawa, Y., Watanabe, M., Nishikawa, K., Higashi, K.: A study combining magic-angle spinning NMR and small-angle X-ray scattering on the interaction in the mixture of poly(benzyl methacrylate) and ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide. Phys. Chem. Chem. Phys., 24, 26575-26582 (2022). https://doi.org/10.1039/D2CP02207A
Morita, T., Okada, H., Yamada, T., Hidaka, R., Ueki, T., Niitsuma, K., Kitazawa, Y., Watanabe, M., Nishikawa, K., Higashi, K.: A study combining magic-angle spinning NMR and small-angle X-ray scattering on the interaction in the mixture of poly(benzyl methacrylate) and ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)amide. Phys. Chem. Chem. Phys., 24, 26575-26582 (2022). https://doi.org/10.1039/D2CP02207A
Yamaguchi, M., Kanazawa, T., Morino, S., Iioka, S., Watanabe, Y., Dohi, N., Higashi, K., Kondo, H., Ishikawa, T.: Increased tropism of extracellular vesicles derived from palmitic acid-treated hepatocytes to activated hepatic stellate cells. Membranes, 12(10), 1023 (2022). https://doi.org/10.3390/membranes12101023
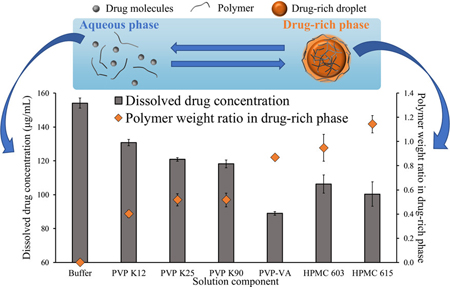
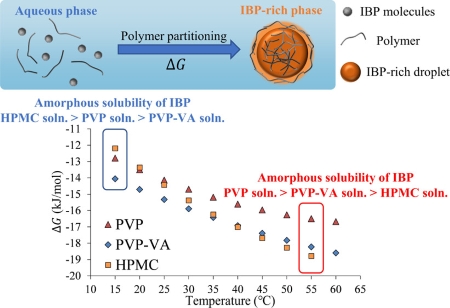 Ueda, K., Higashi, K., Moribe, K., Taylor, L.S. : Variable-temperature NMR analysis of the thermodynamics of polymer partitioning between aqueous and drug-rich phases and its significance for amorphous formulations. Mol. Pharm., 19(1), 100-114 (2022). https://doi.org/10.1021/acs.molpharmaceut.1c00664
Ueda, K., Higashi, K., Moribe, K., Taylor, L.S. : Variable-temperature NMR analysis of the thermodynamics of polymer partitioning between aqueous and drug-rich phases and its significance for amorphous formulations. Mol. Pharm., 19(1), 100-114 (2022). https://doi.org/10.1021/acs.molpharmaceut.1c00664
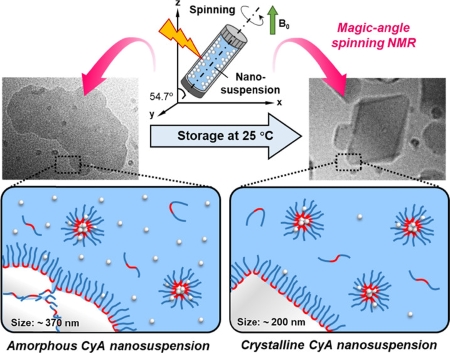 Chen, Z., Higashi, K., Ueda, K., Moribe, K.: Transition from amorphous cyclosporin A nanoparticles to size-reduced stable nanocrystals in a poloxamer 407 solution. Mol. Pharm., 19(1), 188-199 (2022). https://doi.org/10.1021/acs.molpharmaceut.1c00721
Chen, Z., Higashi, K., Ueda, K., Moribe, K.: Transition from amorphous cyclosporin A nanoparticles to size-reduced stable nanocrystals in a poloxamer 407 solution. Mol. Pharm., 19(1), 188-199 (2022). https://doi.org/10.1021/acs.molpharmaceut.1c00721
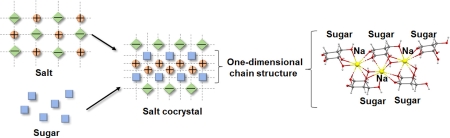 Fujito, T., Oshima, T., Higashi, K., Ueda, K., Ito, M., Masu, H., Noguchi, S., Moribe, K.: Salt cocrystallization of loxoprofen sodium with sugar: Reduction of the propensity for hydrate formation by forming a continuous one-dimensional chain structure of sodium and sugar. Cryst. Growth. Des., 22(2), 1094-1103 (2022). https://doi.org/10.1021/acs.cgd.1c01050
Fujito, T., Oshima, T., Higashi, K., Ueda, K., Ito, M., Masu, H., Noguchi, S., Moribe, K.: Salt cocrystallization of loxoprofen sodium with sugar: Reduction of the propensity for hydrate formation by forming a continuous one-dimensional chain structure of sodium and sugar. Cryst. Growth. Des., 22(2), 1094-1103 (2022). https://doi.org/10.1021/acs.cgd.1c01050
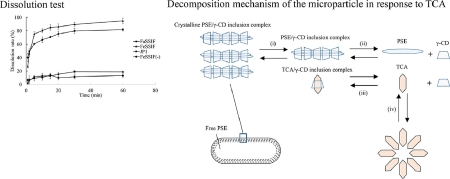 Sasako, H., Koyama, K., Higashi, H., Ueda, K., Ishimoto, A., Moribe, K.: Enteric complex layer-coated controlled release of capsaicin from phytosterol/γ-cyclodextrin microparticles via guest exchange reaction with taurocholic acid. Eur. J. Pharm. Sci., 168, 106038 (2022). https://doi.org/10.1016/j.ejps.2021.106038
Sasako, H., Koyama, K., Higashi, H., Ueda, K., Ishimoto, A., Moribe, K.: Enteric complex layer-coated controlled release of capsaicin from phytosterol/γ-cyclodextrin microparticles via guest exchange reaction with taurocholic acid. Eur. J. Pharm. Sci., 168, 106038 (2022). https://doi.org/10.1016/j.ejps.2021.106038
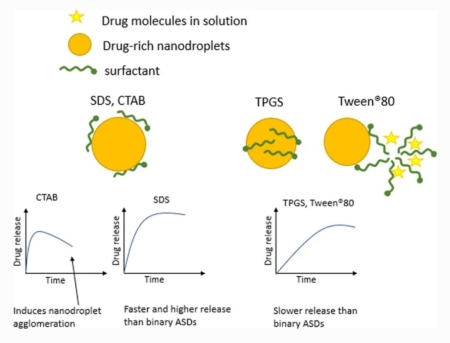 Correa, Soto, C. E., Gao, Y., Indulkar, A. S., Ueda, K., Zhang, G. G., Taylor, L. S.: Impact of surfactants on the performance of clopidogrel-copovidone amorphous solid dispersions: Increased drug loading and stabilization of nanodroplets.. Pharm. Res., 39(1), 167-188 (2022). https://doi.org/10.1007/s11095-021-03159-w
Correa, Soto, C. E., Gao, Y., Indulkar, A. S., Ueda, K., Zhang, G. G., Taylor, L. S.: Impact of surfactants on the performance of clopidogrel-copovidone amorphous solid dispersions: Increased drug loading and stabilization of nanodroplets.. Pharm. Res., 39(1), 167-188 (2022). https://doi.org/10.1007/s11095-021-03159-w
2021
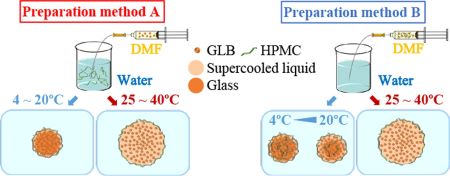 Morikawa, C., Ueda, K., Omori, M., Higashi, K., Moribe, K.: Formation mechanism of amorphous drug nanoparticles using the antisolvent precipitation method elucidated by varying the preparation temperature. Int. J. Pharm., 610, 121210 (2021). https://doi.org/10.1016/j.ijpharm.2021.121210
Morikawa, C., Ueda, K., Omori, M., Higashi, K., Moribe, K.: Formation mechanism of amorphous drug nanoparticles using the antisolvent precipitation method elucidated by varying the preparation temperature. Int. J. Pharm., 610, 121210 (2021). https://doi.org/10.1016/j.ijpharm.2021.121210
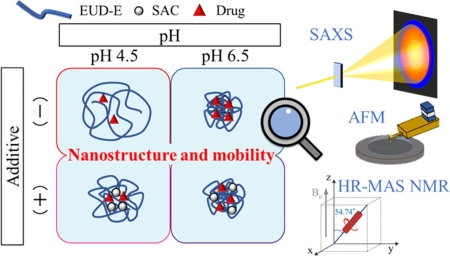 Okamoto, Y., Higashi, K., Morita, T., Ueda, K., Mukaide, S., Takeda, J., Karashima, M., Ikeda, Y., Moribe, K.: Nanostructure and molecular-level characterization of aminoalkyl methacrylate copolymer and the impact on drug solubilization ability. Mol. Pharm., 18(11), 4111-4121 (2021). https://doi.org/10.1021/acs.molpharmaceut.1c00526
Okamoto, Y., Higashi, K., Morita, T., Ueda, K., Mukaide, S., Takeda, J., Karashima, M., Ikeda, Y., Moribe, K.: Nanostructure and molecular-level characterization of aminoalkyl methacrylate copolymer and the impact on drug solubilization ability. Mol. Pharm., 18(11), 4111-4121 (2021). https://doi.org/10.1021/acs.molpharmaceut.1c00526
 Zhao, Z., Higashi, K., Ueda, K., Moribe, K.: Revealing the mechanism of morphological variation of amorphous drug nanoparticles formed by aqueous dispersion of ternary solid dispersion. Int. J. Pharm., 607, 120984 (2021). https://doi.org/10.1016/j.ijpharm.2021.120984
Zhao, Z., Higashi, K., Ueda, K., Moribe, K.: Revealing the mechanism of morphological variation of amorphous drug nanoparticles formed by aqueous dispersion of ternary solid dispersion. Int. J. Pharm., 607, 120984 (2021). https://doi.org/10.1016/j.ijpharm.2021.120984
 Morita, T., Mukaide, S., Chen, Z., Higashi, K., Imamura, H., Moribe, K., Sumi, T.:: Unveiling the interaction potential surface between drug-entrapped polymeric micelles clarifying the high drug nanocarrier efficiency. Nano. Lett., 21(3), 1303-1310 (2021). https://doi.org/10.1021/acs.nanolett.0c03978
Morita, T., Mukaide, S., Chen, Z., Higashi, K., Imamura, H., Moribe, K., Sumi, T.:: Unveiling the interaction potential surface between drug-entrapped polymeric micelles clarifying the high drug nanocarrier efficiency. Nano. Lett., 21(3), 1303-1310 (2021). https://doi.org/10.1021/acs.nanolett.0c03978
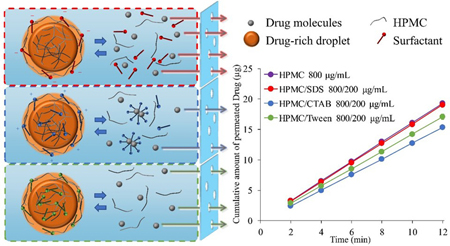 Ueda, K., Taylor, L. S. : Partitioning of surfactant into drug-rich nanodroplets and its impact on drug thermodynamic activity and droplet size. J. Control. Release, 330, 229-243 (2021). https://doi.org/10.1016/j.jconrel.2020.12.018
Ueda, K., Taylor, L. S. : Partitioning of surfactant into drug-rich nanodroplets and its impact on drug thermodynamic activity and droplet size. J. Control. Release, 330, 229-243 (2021). https://doi.org/10.1016/j.jconrel.2020.12.018
 Ueda, K., Moseson, D. E., Pathak, V., Taylor, L. S.: Effect of Polymer Species on Maximum Aqueous Phase Supersaturation Revealed by Quantitative Nuclear Magnetic Resonance Spectroscopy. Mol. Pharm., 18(3), 1344-1355 (2021). https://doi.org/10.1021/acs.molpharmaceut.0c01174
Ueda, K., Moseson, D. E., Pathak, V., Taylor, L. S.: Effect of Polymer Species on Maximum Aqueous Phase Supersaturation Revealed by Quantitative Nuclear Magnetic Resonance Spectroscopy. Mol. Pharm., 18(3), 1344-1355 (2021). https://doi.org/10.1021/acs.molpharmaceut.0c01174
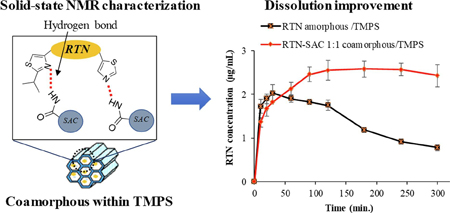 Budiman, A., Higashi, K., Ueda, K., Moribe, K.: Effect of drug-coformer interactions on drug dissolution from a coamorphous in mesoporous silica. Int. J. Pharm., 600, 120492 (2021). https://doi.org/10.1016/j.ijpharm.2021.120492
Budiman, A., Higashi, K., Ueda, K., Moribe, K.: Effect of drug-coformer interactions on drug dissolution from a coamorphous in mesoporous silica. Int. J. Pharm., 600, 120492 (2021). https://doi.org/10.1016/j.ijpharm.2021.120492
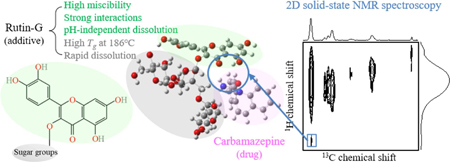 Aoki,C., Ma, Xiaohan., Higashi, K., Ishizuka, Y., Ueda, K., Kadota, K., Fukuzawa, K., Tozuka, Y., Kawakami, K., Yonemochi, E., Moribe, K.: Stabilization mechanism of amorphous carbamazepine by transglycosylated rutin, a non-polymeric amorphous additive with a high glass transition temperature. Int. J. Pharm., 600, 120491 (2021). https://doi.org/10.1016/j.ijpharm.2021.120491
Aoki,C., Ma, Xiaohan., Higashi, K., Ishizuka, Y., Ueda, K., Kadota, K., Fukuzawa, K., Tozuka, Y., Kawakami, K., Yonemochi, E., Moribe, K.: Stabilization mechanism of amorphous carbamazepine by transglycosylated rutin, a non-polymeric amorphous additive with a high glass transition temperature. Int. J. Pharm., 600, 120491 (2021). https://doi.org/10.1016/j.ijpharm.2021.120491
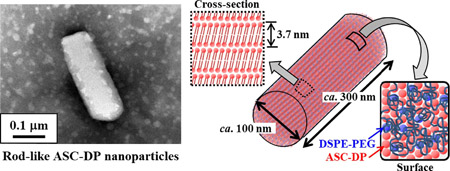 Chen, Z., Higashi, K., Shidara, R., Ueda, K., Morita, T., Limwikrant, W., Yamamoto, K., Moribe, K.: The nanostructure of rod-like ascorbyl dipalmitate nanoparticles stabilized by a small amount of DSPE-PEG. Int. J. Pharm., 602, 120599 (2021). https://doi.org/10.1016/j.ijpharm.2021.120599
Chen, Z., Higashi, K., Shidara, R., Ueda, K., Morita, T., Limwikrant, W., Yamamoto, K., Moribe, K.: The nanostructure of rod-like ascorbyl dipalmitate nanoparticles stabilized by a small amount of DSPE-PEG. Int. J. Pharm., 602, 120599 (2021). https://doi.org/10.1016/j.ijpharm.2021.120599
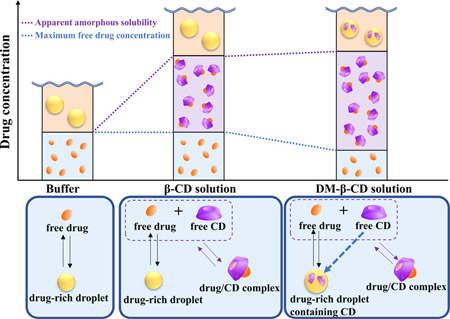 Ueda, K., Higashi, K., Moribe, K.: Amorphous drug solubility and maximum free drug concentrations in cyclodextrin solutions: A quantitative study using NMR diffusometry. Mol. Pharm., 18(7), 2764-2776 (2021). https://doi.org/10.1021/acs.molpharmaceut.1c00311
Ueda, K., Higashi, K., Moribe, K.: Amorphous drug solubility and maximum free drug concentrations in cyclodextrin solutions: A quantitative study using NMR diffusometry. Mol. Pharm., 18(7), 2764-2776 (2021). https://doi.org/10.1021/acs.molpharmaceut.1c00311
2020
 Supasena, W., Muangnoi,C., Thaweesest, W., Songkram,C., Ueda, K., Higashi, K., Moribe, K.,Tanasupawat, S., Rojsitthisak, P.: Enhanced antipsoriatic activity of mycophenolic acid against the TNF-α-induced HaCaT cell proliferation by conjugated poloxamer micelles. J. Pharm. Sci. , 109(2), 1153-1160 (2020). https://doi.org/10.1016/j.xphs.2019.11.010
Supasena, W., Muangnoi,C., Thaweesest, W., Songkram,C., Ueda, K., Higashi, K., Moribe, K.,Tanasupawat, S., Rojsitthisak, P.: Enhanced antipsoriatic activity of mycophenolic acid against the TNF-α-induced HaCaT cell proliferation by conjugated poloxamer micelles. J. Pharm. Sci. , 109(2), 1153-1160 (2020). https://doi.org/10.1016/j.xphs.2019.11.010
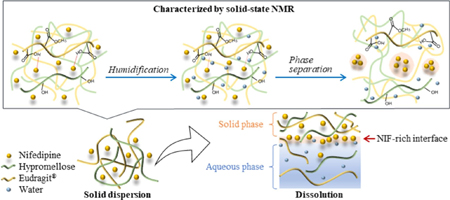 Okada, H., Ueda, K., Yasuda, Y., Higashi, K., Inoue, M., Masataka, Ito., Noguchi, S., Kawakami, K., Moribe, K.: Correlation between drug dissolution and resistance to water-induced phase separation in solid dispersion formulations revealed by solid-state NMR spectroscopy. Int. J. Pharm., 577, 119086 (2020). https://doi.org/10.1016/j.ijpharm.2020.119086
Okada, H., Ueda, K., Yasuda, Y., Higashi, K., Inoue, M., Masataka, Ito., Noguchi, S., Kawakami, K., Moribe, K.: Correlation between drug dissolution and resistance to water-induced phase separation in solid dispersion formulations revealed by solid-state NMR spectroscopy. Int. J. Pharm., 577, 119086 (2020). https://doi.org/10.1016/j.ijpharm.2020.119086
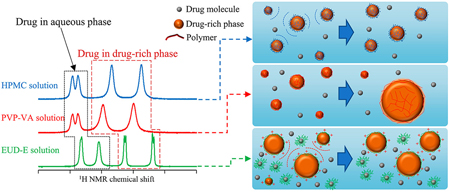 Ueda, K., Taylor, L. S. : Polymer type impacts amorphous solubility and drug-rich phase colloidal stability: A mechanistic study using nuclear magnetic resonance spectroscopy. Mol. Pharm., 17(4), 1352-1362 (2020). https://doi.org/10.1021/acs.molpharmaceut.0c00061
Ueda, K., Taylor, L. S. : Polymer type impacts amorphous solubility and drug-rich phase colloidal stability: A mechanistic study using nuclear magnetic resonance spectroscopy. Mol. Pharm., 17(4), 1352-1362 (2020). https://doi.org/10.1021/acs.molpharmaceut.0c00061
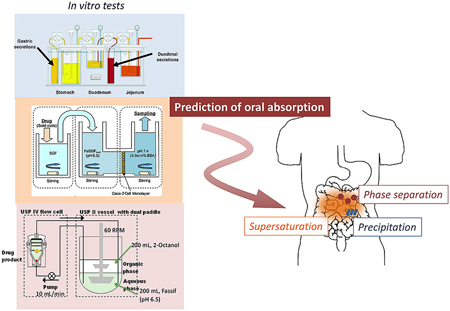 Hens, B., Kataoka, M., Ueda, K., Gao, P., Tsume, Y., Augustijnsp, P., Kawakami, K., Yamashita, S.: Biopredictive in vitro testing methods to assess intestinal drug absorption from supersaturating dosage forms. J. Drug. Deliv. Sci. Technol., 56, 101275 (2020). https://doi.org/10.1016/j.jddst.2019.101275
Hens, B., Kataoka, M., Ueda, K., Gao, P., Tsume, Y., Augustijnsp, P., Kawakami, K., Yamashita, S.: Biopredictive in vitro testing methods to assess intestinal drug absorption from supersaturating dosage forms. J. Drug. Deliv. Sci. Technol., 56, 101275 (2020). https://doi.org/10.1016/j.jddst.2019.101275
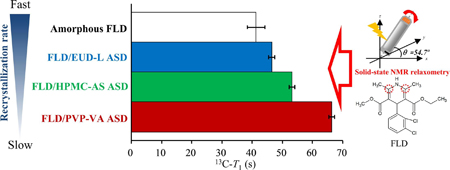 Ueda, K., Okada, H., Zhao, Z., Higashi, K., Moribe, K.: Application of solid-state 13C relaxation time to prediction of the recrystallization inhibition strength of polymers on amorphous felodipine at low polymer loading.. Int. J. Pharm., 581, 119300 (2020). https://doi.org/10.1016/j.ijpharm.2020.119300
Ueda, K., Okada, H., Zhao, Z., Higashi, K., Moribe, K.: Application of solid-state 13C relaxation time to prediction of the recrystallization inhibition strength of polymers on amorphous felodipine at low polymer loading.. Int. J. Pharm., 581, 119300 (2020). https://doi.org/10.1016/j.ijpharm.2020.119300
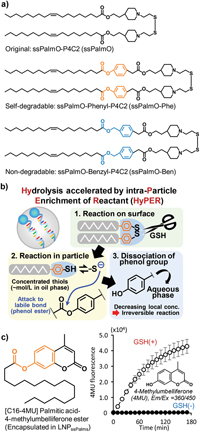 Tanaka, H., Takahashi, T., Konishi, M., Takata, N., Gomi, M., Shirane, D., Miyama, R., Hagiwara, S., Yamasaki, Y., Sakurai, Y., Ueda, K., Higashi, K., Moribe, K., Shinsho, E., Nishida, R., Fukuzawa, K., Yonemochi, E., Okuwaki, K., Mochizuki, Y., Nakai, Y., Tange, K., Yoshioka, H., Tamagawa, S., Akita, H.: Self‐degradable lipid‐like materials based on “hydrolysis accelerated by the intra‐particle enrichment of reactant (HyPER)” for messenger RNA delivery. Adv. Funct. Mater., 30, 1920575 (2020). https://doi.org/10.1002/adfm.201910575
Tanaka, H., Takahashi, T., Konishi, M., Takata, N., Gomi, M., Shirane, D., Miyama, R., Hagiwara, S., Yamasaki, Y., Sakurai, Y., Ueda, K., Higashi, K., Moribe, K., Shinsho, E., Nishida, R., Fukuzawa, K., Yonemochi, E., Okuwaki, K., Mochizuki, Y., Nakai, Y., Tange, K., Yoshioka, H., Tamagawa, S., Akita, H.: Self‐degradable lipid‐like materials based on “hydrolysis accelerated by the intra‐particle enrichment of reactant (HyPER)” for messenger RNA delivery. Adv. Funct. Mater., 30, 1920575 (2020). https://doi.org/10.1002/adfm.201910575
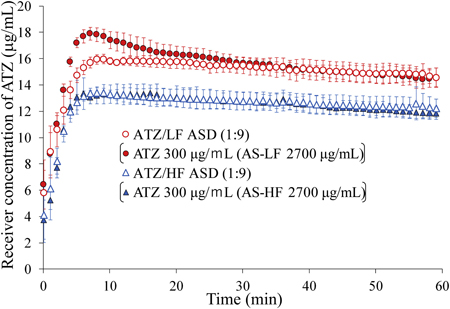 Ueda, K., Hate, S. S., Taylor, L. S.: Impact of hypromellose acetate succinate grade on drug amorphous solubility and in vitro membrane transport. J. Pharm. Sci. , 190(8), 2464-2473 (2020). https://doi.org/10.1016/j.xphs.2020.04.014
Ueda, K., Hate, S. S., Taylor, L. S.: Impact of hypromellose acetate succinate grade on drug amorphous solubility and in vitro membrane transport. J. Pharm. Sci. , 190(8), 2464-2473 (2020). https://doi.org/10.1016/j.xphs.2020.04.014
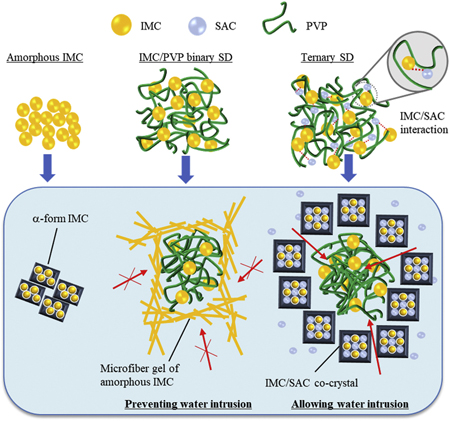 Kosaka, M., Higashi, K., Nishimura, M., Ueda, K., Moribem K.: Clarification of the dissolution mechanism of an indomethacin/ saccharin/polyvinylpyrrolidone ternary solid dispersion by NMR spectroscopy. J. Pharm. Sci. , 109(12), 3617-3624 (2020). https://doi.org/10.1016/j.xphs.2020.09.009
Kosaka, M., Higashi, K., Nishimura, M., Ueda, K., Moribem K.: Clarification of the dissolution mechanism of an indomethacin/ saccharin/polyvinylpyrrolidone ternary solid dispersion by NMR spectroscopy. J. Pharm. Sci. , 109(12), 3617-3624 (2020). https://doi.org/10.1016/j.xphs.2020.09.009
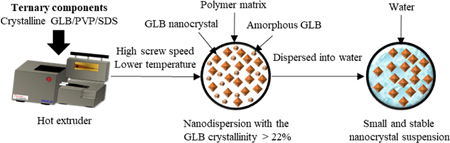 Omagari, K., Ueda, K., Zhijing, Z., Higashi, K., Inoue, M., Fukami, T., Moribe, K.: Mechanistic study of preparation of drug/polymer/surfactant ternary hot extrudates to obtain small and stable drug nanocrystal suspensions. Int. J. Pharm., 591, 120003 (2020). https://doi.org/10.1016/j.ijpharm.2020.120003
Omagari, K., Ueda, K., Zhijing, Z., Higashi, K., Inoue, M., Fukami, T., Moribe, K.: Mechanistic study of preparation of drug/polymer/surfactant ternary hot extrudates to obtain small and stable drug nanocrystal suspensions. Int. J. Pharm., 591, 120003 (2020). https://doi.org/10.1016/j.ijpharm.2020.120003
2019
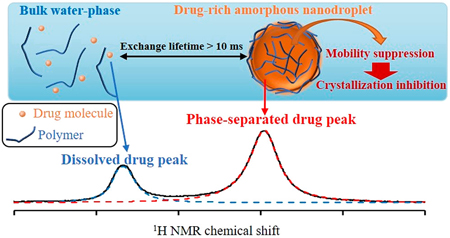 Ueda, K., Yamamoto, N., Higashi, K., Moribe, K.: Molecular mobility suppression of ibuprofen-rich amorphous nanodroplets by HPMC revealed by NMR relaxometry and its significance with respect to crystallization inhibition. Mol. Pharm., 16(12), 4968-4977 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00840
Ueda, K., Yamamoto, N., Higashi, K., Moribe, K.: Molecular mobility suppression of ibuprofen-rich amorphous nanodroplets by HPMC revealed by NMR relaxometry and its significance with respect to crystallization inhibition. Mol. Pharm., 16(12), 4968-4977 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00840
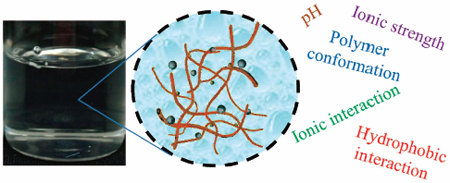 Higashi, K., Ueda, K., Moribe, K.: Intermolecular interactions between drugs and aminoalkyl methacrylate copolymer in solution to enhance the concentration of poorly water-soluble drugs. Chem. Pharm. Bull., 67 (9), 906-914 (2019). https://doi.org/10.1248/cpb.c18-00849
Higashi, K., Ueda, K., Moribe, K.: Intermolecular interactions between drugs and aminoalkyl methacrylate copolymer in solution to enhance the concentration of poorly water-soluble drugs. Chem. Pharm. Bull., 67 (9), 906-914 (2019). https://doi.org/10.1248/cpb.c18-00849
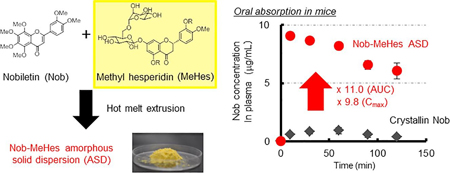 Iwashita, M., Hashizume, K., Umehara, M., Ishigami, T., Onishi, S., Yamamoto, M., Higashi, K., Moribe, K.: Development of nobiletin?methyl hesperidin amorphous solid dispersion: Novel application of methyl hesperidin as an excipient for hot-melt extrusion. Int. J. Pharm., 558(10), 215-224 (2019). https://doi.org/10.1016/j.ijpharm.2018.12.092
Iwashita, M., Hashizume, K., Umehara, M., Ishigami, T., Onishi, S., Yamamoto, M., Higashi, K., Moribe, K.: Development of nobiletin?methyl hesperidin amorphous solid dispersion: Novel application of methyl hesperidin as an excipient for hot-melt extrusion. Int. J. Pharm., 558(10), 215-224 (2019). https://doi.org/10.1016/j.ijpharm.2018.12.092
 Shinozaki, T., Ono, M., Higashi, K., Moribe, K.: A novel drug-drug co-crystal of levofloxacin and metacetamol: Reduced hygroscopicity and improved photostability of levofloxacin. J. Pharm. Sci., 108 (7), 2383-2390 (2019). https://doi.org/10.1016/j.xphs.2019.02.014
Shinozaki, T., Ono, M., Higashi, K., Moribe, K.: A novel drug-drug co-crystal of levofloxacin and metacetamol: Reduced hygroscopicity and improved photostability of levofloxacin. J. Pharm. Sci., 108 (7), 2383-2390 (2019). https://doi.org/10.1016/j.xphs.2019.02.014
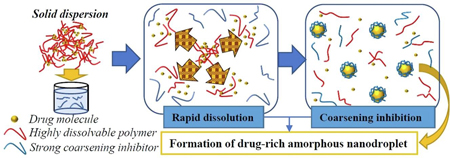 Ueda, K., Higashi, K., Moribe, K.: Mechanistic elucidation of formation of drug-rich amorphous nanodroplets by dissolution of the solid dispersion formulation. Int. J. Pharm., 561(20), 82-92 (2019). https://doi.org/10.1016/j.ijpharm.2019.02.034
Ueda, K., Higashi, K., Moribe, K.: Mechanistic elucidation of formation of drug-rich amorphous nanodroplets by dissolution of the solid dispersion formulation. Int. J. Pharm., 561(20), 82-92 (2019). https://doi.org/10.1016/j.ijpharm.2019.02.034
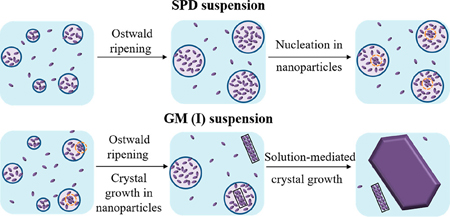 Zhao, Z., Katai, H., Higashi, K., Ueda, K.,Kawakami, K., Moribe, K.: Cryo-TEM and AFM observation of the time-dependent evolution of amorphous probucol nanoparticles formed by the aqueous dispersion of ternary solid dispersions. Mol. Pharm., 16(5), 2184-2198 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00158
Zhao, Z., Katai, H., Higashi, K., Ueda, K.,Kawakami, K., Moribe, K.: Cryo-TEM and AFM observation of the time-dependent evolution of amorphous probucol nanoparticles formed by the aqueous dispersion of ternary solid dispersions. Mol. Pharm., 16(5), 2184-2198 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00158
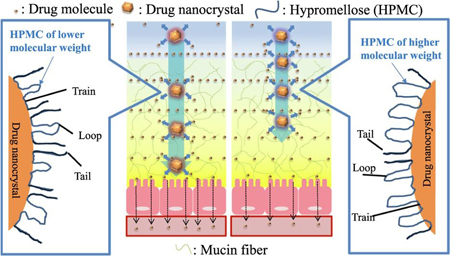 Ueda, K., Iwai, T., Sunazuka, Y., Chen, Z., Kato, N., Higashi, K., Moribe, K.: Effect of molecular weight of hypromellose on mucin diffusion and oral absorption behavior of fenofibrate nanocrystal. Int. J. Pharm., 564(10), 39-47 (2019). https://doi.org/10.1016/j.ijpharm.2019.04.033
Ueda, K., Iwai, T., Sunazuka, Y., Chen, Z., Kato, N., Higashi, K., Moribe, K.: Effect of molecular weight of hypromellose on mucin diffusion and oral absorption behavior of fenofibrate nanocrystal. Int. J. Pharm., 564(10), 39-47 (2019). https://doi.org/10.1016/j.ijpharm.2019.04.033
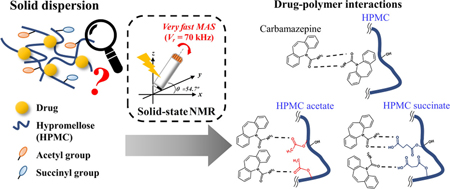 Ishizuka, Y., Ueda, K., Okada, H., Takeda, J., Karashima, M., Yazawa, K., Higashi, K., Kawakami, K., Ikeda, Y., Moribe, K.:Effect of drug-polymer interactions through hypromellose acetate succinate substituents on the physical stability on solid dispersions studied by Fourier-transform infrared and solid-state nuclear magnetic resonance. Mol. Pharm., 16(6), 2785-2794 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00301
Ishizuka, Y., Ueda, K., Okada, H., Takeda, J., Karashima, M., Yazawa, K., Higashi, K., Kawakami, K., Ikeda, Y., Moribe, K.:Effect of drug-polymer interactions through hypromellose acetate succinate substituents on the physical stability on solid dispersions studied by Fourier-transform infrared and solid-state nuclear magnetic resonance. Mol. Pharm., 16(6), 2785-2794 (2019). https://doi.org/10.1021/acs.molpharmaceut.9b00301
2018
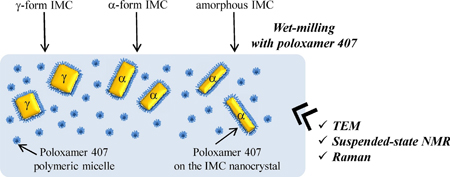 Kuroiwa, Y., Higashi, K., Ueda, K., Yamamoto, K., Moribe, K.: Nano-scale and molecular-level understanding of wet-milled indomethacin/poloxamer 407 nanosuspension with TEM, suspended-state NMR, and Raman measurements. Int. J. Pharm., 537 (1-2), 30-39 (2018). https://doi.org/10.1016/j.ijpharm.2017.12.028
Kuroiwa, Y., Higashi, K., Ueda, K., Yamamoto, K., Moribe, K.: Nano-scale and molecular-level understanding of wet-milled indomethacin/poloxamer 407 nanosuspension with TEM, suspended-state NMR, and Raman measurements. Int. J. Pharm., 537 (1-2), 30-39 (2018). https://doi.org/10.1016/j.ijpharm.2017.12.028
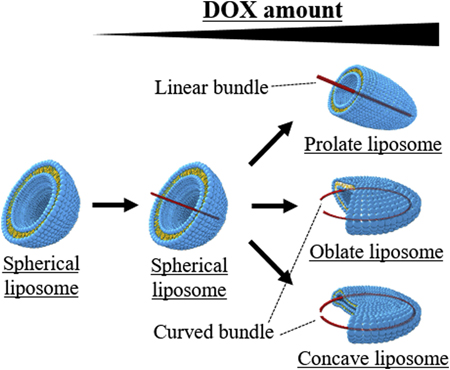 Takahashi, N., Higashi, K., Ueda, K., Yamamoto, K., Moribe, K.: Determination of nonspherical morphology of doxorubicin-loaded liposomes by atomic force microscopy. J. Pharm. Sci., 107 (2), 717-726 (2018). https://doi.org/10.1016/j.xphs.2017.10.009
Takahashi, N., Higashi, K., Ueda, K., Yamamoto, K., Moribe, K.: Determination of nonspherical morphology of doxorubicin-loaded liposomes by atomic force microscopy. J. Pharm. Sci., 107 (2), 717-726 (2018). https://doi.org/10.1016/j.xphs.2017.10.009
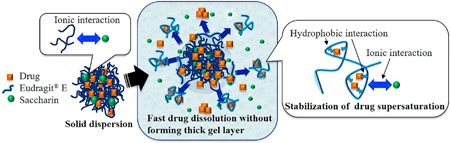 Ueda, K., Kanaya, H., Higashi, K., Yamamoto, K., Moribe, K.: Molecular-level elucidation of saccharin-assisted rapid dissolution and high supersaturation level of drug from Eudragit® E solid dispersion. Int. J. Pharm., 538(1-2), 57-64 (2018). https://doi.org/10.1016/j.ijpharm.2017.12.050
Ueda, K., Kanaya, H., Higashi, K., Yamamoto, K., Moribe, K.: Molecular-level elucidation of saccharin-assisted rapid dissolution and high supersaturation level of drug from Eudragit® E solid dispersion. Int. J. Pharm., 538(1-2), 57-64 (2018). https://doi.org/10.1016/j.ijpharm.2017.12.050
Morio, H., Sun, Y., Harada, M., Ide, H., Shimozato, O., Zhou, X. Higashi, K., Yuki, R., Yamaguchi, N., Hofbauer, J.P., Guttmann-Gruber, C., Anzai, N., Akita, H., Chiba, K., Furihata, T.: Cancer-type OATP1B3 mRNA in extracellular vesicles as a promising candidate for a serum-based colorectal cancer biomarker. Biol. Pharm. Bull., 41(3), 445-449 (2018). https://doi.org/10.1248/bpb.b17-00743
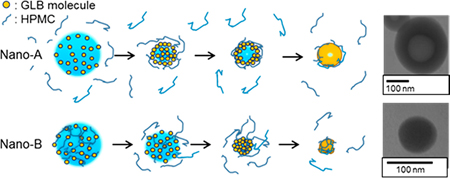 Yonashiro, H., Higashi, K., Morikawa, C., Ueda, K., Itoh, T., Ito, M., Masu, H., Noguchi, S., Moribe, K.: Morphological and physicochemical evaluation of two distinct glibenclamide/hypromellose amorphous nanoparticles prepared by the antisolvent method. Mol. Pharm., 15(4), 1587-1597 (2018). https://doi.org/10.1021/acs.molpharmaceut.7b01122
Yonashiro, H., Higashi, K., Morikawa, C., Ueda, K., Itoh, T., Ito, M., Masu, H., Noguchi, S., Moribe, K.: Morphological and physicochemical evaluation of two distinct glibenclamide/hypromellose amorphous nanoparticles prepared by the antisolvent method. Mol. Pharm., 15(4), 1587-1597 (2018). https://doi.org/10.1021/acs.molpharmaceut.7b01122
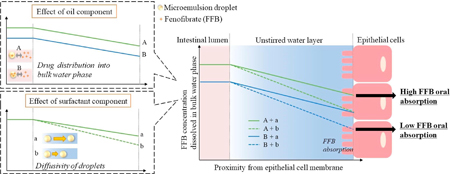 Sunazuka, Y., Ueda, K., Higashi, K., Tanaka, Y., Moribe, K.: Combined effects of the drug distribution and mucus diffusion properties of self-microemulsifying drug delivery systems on the oral absorption of fenofibrate. Int. J. Pharm., 546 (1), 263-271(2018). https://doi.org/10.1016/j.ijpharm.2018.05.031
Sunazuka, Y., Ueda, K., Higashi, K., Tanaka, Y., Moribe, K.: Combined effects of the drug distribution and mucus diffusion properties of self-microemulsifying drug delivery systems on the oral absorption of fenofibrate. Int. J. Pharm., 546 (1), 263-271(2018). https://doi.org/10.1016/j.ijpharm.2018.05.031
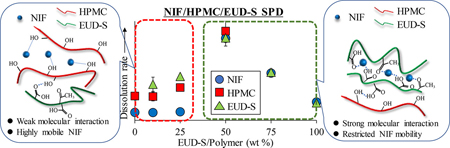 Ueda, K., Yamazoe, C., Yasuda, Y., Higashi, K., Kawakami, K., Moribe, K.: Mechanism of enhanced nifedipine dissolution by polymer-blended solid dispersion through molecular-level characterization. Mol. Pharm., 15(9),4099-4109 (2018). https://doi.org/10.1021/acs.molpharmaceut.8b00523
Ueda, K., Yamazoe, C., Yasuda, Y., Higashi, K., Kawakami, K., Moribe, K.: Mechanism of enhanced nifedipine dissolution by polymer-blended solid dispersion through molecular-level characterization. Mol. Pharm., 15(9),4099-4109 (2018). https://doi.org/10.1021/acs.molpharmaceut.8b00523
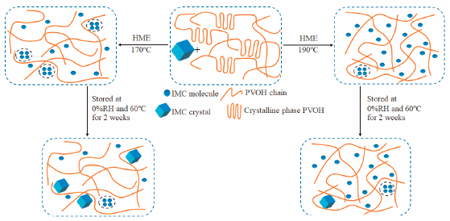 Benjasirimongkol, P., Ueda, K., Higashi, K., Sriamornsak, P., Moribe, K.: An insight into stabilization mechanism of a solid dispersion of indomethacin/partially hydrolyzed polyvinyl alcohol prepared by hot-melt extrusion. Chem. Pharm. Bull.,66 (9), 859-865(2018). https://doi.org/10.1248/cpb.c18-00362
Benjasirimongkol, P., Ueda, K., Higashi, K., Sriamornsak, P., Moribe, K.: An insight into stabilization mechanism of a solid dispersion of indomethacin/partially hydrolyzed polyvinyl alcohol prepared by hot-melt extrusion. Chem. Pharm. Bull.,66 (9), 859-865(2018). https://doi.org/10.1248/cpb.c18-00362
2017
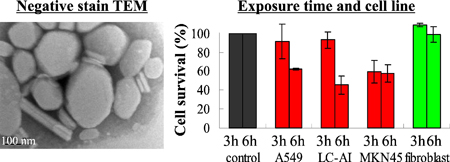 K. Higashi, F. Mibu, K. Saito, W. Limwikrant, K. Yamamoto, K. Moribe. Composition-dependent structural changes and antitumor activity of ASC-DP/DSPE-PEG nanoparticles. Eur. J. Pharm. Sci., 99, 24-31 (2017). https://doi.org/10.1016/j.ejps.2016.11.029
K. Higashi, F. Mibu, K. Saito, W. Limwikrant, K. Yamamoto, K. Moribe. Composition-dependent structural changes and antitumor activity of ASC-DP/DSPE-PEG nanoparticles. Eur. J. Pharm. Sci., 99, 24-31 (2017). https://doi.org/10.1016/j.ejps.2016.11.029
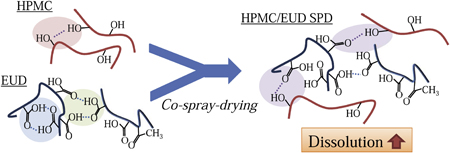 N. Ohyagi, K. Ueda, K. Higashi, K. Yamamoto, K. Kawakami, K. Moribe. Synergetic role of hypromellose and methacrylic acid copolymer in the dissolution improvement of amorphous solid dispersions. J. Pharm. Sci., 106 (4), 1042-1050 (2017). https://doi.org/10.1016/j.xphs.2016.12.005
N. Ohyagi, K. Ueda, K. Higashi, K. Yamamoto, K. Kawakami, K. Moribe. Synergetic role of hypromellose and methacrylic acid copolymer in the dissolution improvement of amorphous solid dispersions. J. Pharm. Sci., 106 (4), 1042-1050 (2017). https://doi.org/10.1016/j.xphs.2016.12.005
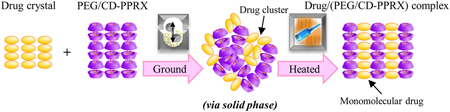 M. Ogawa, K. Higashi, S. Namiki, N. Liu, K. Ueda, W. Limwikrant, K. Yamamoto, K. Moribe. A solid-phase mediated methodology to incorporate drug into intermolecular spaces of CD columns in the PEG/CD-polypseudorotaxanes by cogrinding and subsequent heating. Cryst. Growth Des., 17 (3), 1055-1068 (2017). https://doi.org/10.1021/acs.cgd.6b01410
M. Ogawa, K. Higashi, S. Namiki, N. Liu, K. Ueda, W. Limwikrant, K. Yamamoto, K. Moribe. A solid-phase mediated methodology to incorporate drug into intermolecular spaces of CD columns in the PEG/CD-polypseudorotaxanes by cogrinding and subsequent heating. Cryst. Growth Des., 17 (3), 1055-1068 (2017). https://doi.org/10.1021/acs.cgd.6b01410
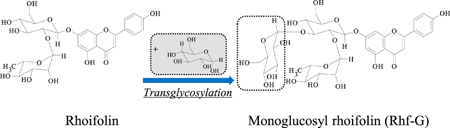 C. Aoki, Y. Takeuchi, K. Higashi, Y. Okamoto, A. Nakanishi, M. Tandia, J. Uzawa, K. Ueda, K. Moribe. Structural elucidation of a novel transglycosylated compound α-glucosyl rhoifolin and of α-glucosyl rutin by NMR spectroscopy. Carbohydr. Res., 443-444 (18), 37-41 (2017). https://doi.org/10.1016/j.carres.2017.03.011
C. Aoki, Y. Takeuchi, K. Higashi, Y. Okamoto, A. Nakanishi, M. Tandia, J. Uzawa, K. Ueda, K. Moribe. Structural elucidation of a novel transglycosylated compound α-glucosyl rhoifolin and of α-glucosyl rutin by NMR spectroscopy. Carbohydr. Res., 443-444 (18), 37-41 (2017). https://doi.org/10.1016/j.carres.2017.03.011
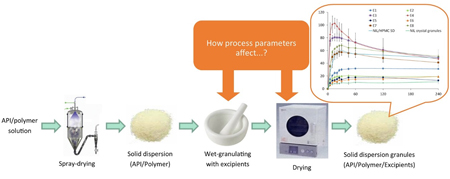 R. Kinoshita, T. Ohta, K. Shiraki, K. Higashi, K. Moribe. Effects of wet-granulation process parameters on the dissolution and physical stability of a solid dispersion. Int. J. Pharm., 524 (1), 304-311 (2017). https://doi.org/10.1016/j.ijpharm.2017.04.007
R. Kinoshita, T. Ohta, K. Shiraki, K. Higashi, K. Moribe. Effects of wet-granulation process parameters on the dissolution and physical stability of a solid dispersion. Int. J. Pharm., 524 (1), 304-311 (2017). https://doi.org/10.1016/j.ijpharm.2017.04.007
M. Kitajima, A. Morita, S. Endo, N. Kogure, K. Higashi, K. Moribe, H. Takayama. Design and synthesis of 4-chlorocolchicine-derived prodrug capable of forming nanoparticles by self-assembly. Heterocycles, 95 (1), 181-186 (2017). https://doi.org/10.3987/COM-16-S(S)51
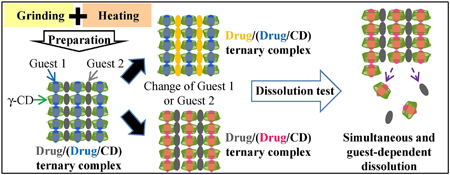 N. Liu, K. Higashi, K. Ueda, K. Moribe. Effect of guest drug character encapsulated in the cavity and intermolecular spaces of γ-cyclodextrins on the dissolution property of ternary γ-cyclodextrin complex. Int. J. Pharm., 531 (2), 543-549 (2017). https://doi.org/10.1016/j.ijpharm.2017.04.049
N. Liu, K. Higashi, K. Ueda, K. Moribe. Effect of guest drug character encapsulated in the cavity and intermolecular spaces of γ-cyclodextrins on the dissolution property of ternary γ-cyclodextrin complex. Int. J. Pharm., 531 (2), 543-549 (2017). https://doi.org/10.1016/j.ijpharm.2017.04.049
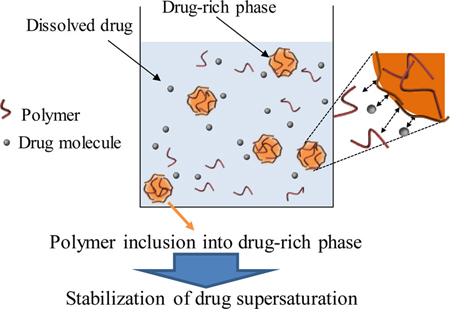 K. Ueda, K. Higashi, K. Moribe. Direct NMR monitoring of phase separation behavior of highly supersaturated nifedipine solution stabilized with hypromellose derivatives. Mol. Pharm., 14 (7), 2314–2322 (2017). https://doi.org/10.1021/acs.molpharmaceut.7b00178
K. Ueda, K. Higashi, K. Moribe. Direct NMR monitoring of phase separation behavior of highly supersaturated nifedipine solution stabilized with hypromellose derivatives. Mol. Pharm., 14 (7), 2314–2322 (2017). https://doi.org/10.1021/acs.molpharmaceut.7b00178
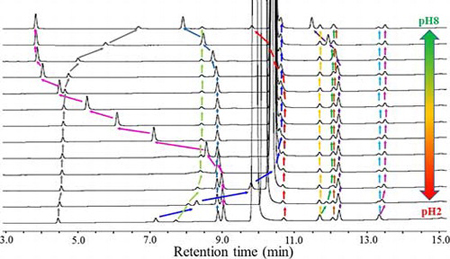 S. Furukawa, Y. Hirakura, K. Moribe, Continuous screening of analytical parameters facilitates efficient development of HPLC methods required for impurity profiling. J. Liq. Chromatogr. Relat. Technol., 40 (11), 564-575 (2017). https://doi.org/10.1080/10826076.2017.1334214
S. Furukawa, Y. Hirakura, K. Moribe, Continuous screening of analytical parameters facilitates efficient development of HPLC methods required for impurity profiling. J. Liq. Chromatogr. Relat. Technol., 40 (11), 564-575 (2017). https://doi.org/10.1080/10826076.2017.1334214
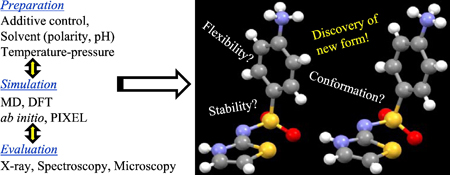 K. Higashi, K. Ueda, K. Moribe. Recent progress of structural study of polymorphic pharmaceutical drugs. Adv. Drug Deliv. Rev., 117, 71-85 (2017). https://doi.org/10.1016/j.addr.2016.12.001
K. Higashi, K. Ueda, K. Moribe. Recent progress of structural study of polymorphic pharmaceutical drugs. Adv. Drug Deliv. Rev., 117, 71-85 (2017). https://doi.org/10.1016/j.addr.2016.12.001
2016
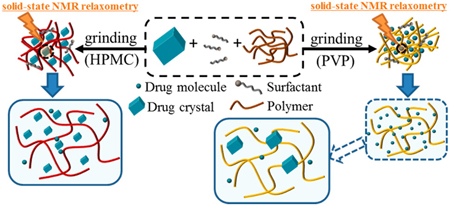 K. Ueda, K. Higashi, K. Moribe. Application of solid-state NMR relaxometry for characterization and formulation optimization of grinding-induced drug nanoparticle. Mol. Pharm., 13 (3), 852-862 (2016). https://doi.org/10.1021/acs.molpharmaceut.5b00781
K. Ueda, K. Higashi, K. Moribe. Application of solid-state NMR relaxometry for characterization and formulation optimization of grinding-induced drug nanoparticle. Mol. Pharm., 13 (3), 852-862 (2016). https://doi.org/10.1021/acs.molpharmaceut.5b00781
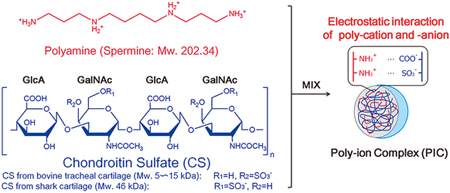 D. Ge, K. Higashi, D. Ito, K. Nagano, R. Ishikawa, Y. Terui, K. Higashi, K. Moribe, J.R. Linhardt, T. Toida. Poly-ion complex of chondroitin sulfate and spermine and its effect on oral chondroitin sulfate bioavailability. Chem. Pharm. Bull., 64(5), 390-398 (2016). https://doi.org/10.1248/cpb.c15-00940
D. Ge, K. Higashi, D. Ito, K. Nagano, R. Ishikawa, Y. Terui, K. Higashi, K. Moribe, J.R. Linhardt, T. Toida. Poly-ion complex of chondroitin sulfate and spermine and its effect on oral chondroitin sulfate bioavailability. Chem. Pharm. Bull., 64(5), 390-398 (2016). https://doi.org/10.1248/cpb.c15-00940
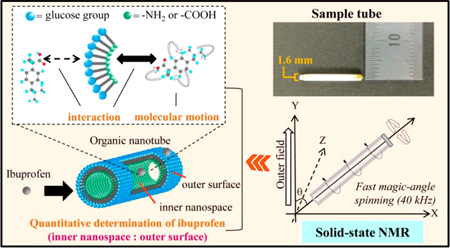 N. Liu, K. Higashi, J. Kikuchi, S. Ando, N. Kameta, W. Ding, M. Masuda, T. Shimizu, K. Ueda, K. Yamamoto, K. Moribe K. Molecular-level understanding of the encapsulation and dissolution of poorly water-soluble ibuprofen by functionalized organic nanotubes using solid-state NMR spectroscopy. J. Phys. Chem. B, 120 (19), 4496-4507 (2016). https://doi.org/10.1021/acs.jpcb.6b00939
N. Liu, K. Higashi, J. Kikuchi, S. Ando, N. Kameta, W. Ding, M. Masuda, T. Shimizu, K. Ueda, K. Yamamoto, K. Moribe K. Molecular-level understanding of the encapsulation and dissolution of poorly water-soluble ibuprofen by functionalized organic nanotubes using solid-state NMR spectroscopy. J. Phys. Chem. B, 120 (19), 4496-4507 (2016). https://doi.org/10.1021/acs.jpcb.6b00939
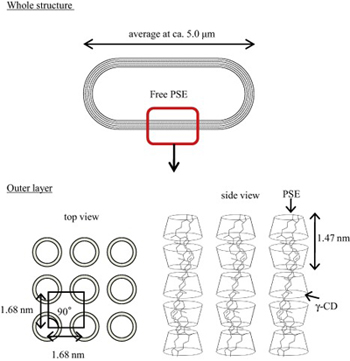 H. Sasako, F. Kihara, K. Koyama, K. Higashi, K. Yamamoto, K. Moribe. A novel capsule-like structure of micro-sized particles formed by phytosterol ester and γ-cyclodextrin in water. Food Chem., 210, 269-275 (2016). https://doi.org/10.1016/j.foodchem.2016.04.103
H. Sasako, F. Kihara, K. Koyama, K. Higashi, K. Yamamoto, K. Moribe. A novel capsule-like structure of micro-sized particles formed by phytosterol ester and γ-cyclodextrin in water. Food Chem., 210, 269-275 (2016). https://doi.org/10.1016/j.foodchem.2016.04.103
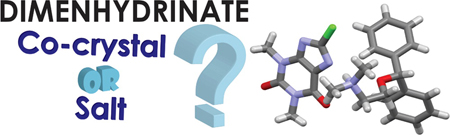 OD. Putra, T. Yoshida, D. Umeda, K. Higashi, H. Uekusa, E. Yonemochi. Crystal structure determination of dimenhydrinate after more than 60 years: Solving salt-cocrystal ambiguity via solid-state characterizations and solubility study. Cryst. Growth Des., 16 (9), 5223-5229 (2016). https://doi.org/10.1021/acs.cgd.6b00771
OD. Putra, T. Yoshida, D. Umeda, K. Higashi, H. Uekusa, E. Yonemochi. Crystal structure determination of dimenhydrinate after more than 60 years: Solving salt-cocrystal ambiguity via solid-state characterizations and solubility study. Cryst. Growth Des., 16 (9), 5223-5229 (2016). https://doi.org/10.1021/acs.cgd.6b00771
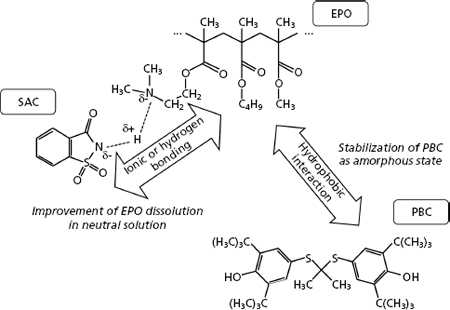 K. Higashi, A. Seo, K. Egami, N. Otsuka, W. Limwikrant, K. Yamamoto, K. Moribe. Mechanistic insight into the dramatic improvement of probucol dissolution in neutral solutions by solid dispersion in Eudragit® E PO with saccharin. J. Pharm. Pharmacol., 68(5), 655-664 (2016).https://doi.org/10.1111/jphp.12469
K. Higashi, A. Seo, K. Egami, N. Otsuka, W. Limwikrant, K. Yamamoto, K. Moribe. Mechanistic insight into the dramatic improvement of probucol dissolution in neutral solutions by solid dispersion in Eudragit® E PO with saccharin. J. Pharm. Pharmacol., 68(5), 655-664 (2016).https://doi.org/10.1111/jphp.12469
2015
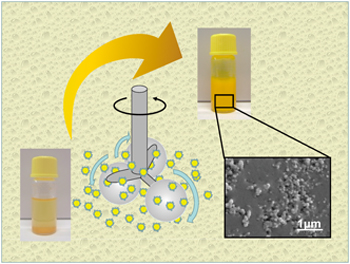 T. Sato, H. Takeuchi,T. Sakurai, K. Tanaka, K. Matsuki, K. Higashi, K. Moribe,K. Yamamoto. Characterization of a riboflavin non-aqueous nanosuspension prepared by bead milling for cutaneous application. Chem. Pharm. Bull., 63 (2), 88-94 (2015). https://doi.org/10.1248/cpb.c14-00641
T. Sato, H. Takeuchi,T. Sakurai, K. Tanaka, K. Matsuki, K. Higashi, K. Moribe,K. Yamamoto. Characterization of a riboflavin non-aqueous nanosuspension prepared by bead milling for cutaneous application. Chem. Pharm. Bull., 63 (2), 88-94 (2015). https://doi.org/10.1248/cpb.c14-00641
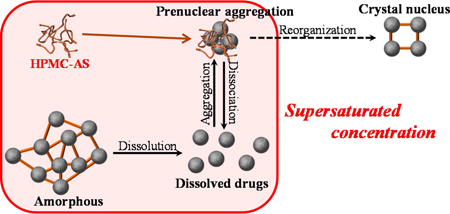 K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. Equilibrium state at supersaturated drug concentration achieved by hydroxypropyl methylcellulose acetate succinate: molecular characterization using 1H NMR technique. Mol. Pharm., 12 (4), 1096-1104 (2015). https://doi.org/10.1021/mp500588x
K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. Equilibrium state at supersaturated drug concentration achieved by hydroxypropyl methylcellulose acetate succinate: molecular characterization using 1H NMR technique. Mol. Pharm., 12 (4), 1096-1104 (2015). https://doi.org/10.1021/mp500588x
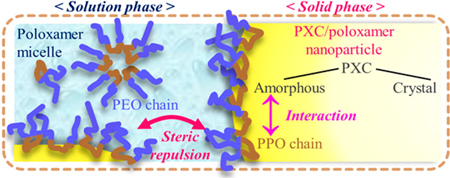 Y. Hasegawa, K. Higashi, K. Yamamoto, K. Moribe. Direct evaluation of molecular states of piroxicam/poloxamer nanosuspension by suspended-state NMR and Raman spectroscopies. Mol. Pharm., 12 (5), 1564-1572 (2015). https://doi.org/10.1021/mp500872g
Y. Hasegawa, K. Higashi, K. Yamamoto, K. Moribe. Direct evaluation of molecular states of piroxicam/poloxamer nanosuspension by suspended-state NMR and Raman spectroscopies. Mol. Pharm., 12 (5), 1564-1572 (2015). https://doi.org/10.1021/mp500872g
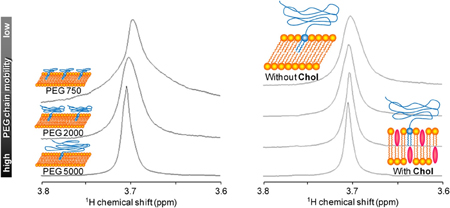 K. Abe, K. Higashi, K. Watabe, K. Kobayashi, W. Limwikrant, K. Yamamoto, K. Moribe. Effects of the PEG molecular weight of a PEG-lipid and cholesterol on PEG chain flexibility on liposome surfaces. Colloids Surf. A., 474 (5), 63-70 (2015). https://doi.org/10.1016/j.colsurfa.2015.03.006
K. Abe, K. Higashi, K. Watabe, K. Kobayashi, W. Limwikrant, K. Yamamoto, K. Moribe. Effects of the PEG molecular weight of a PEG-lipid and cholesterol on PEG chain flexibility on liposome surfaces. Colloids Surf. A., 474 (5), 63-70 (2015). https://doi.org/10.1016/j.colsurfa.2015.03.006
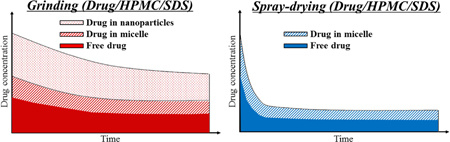 K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. In situ molecular elucidation of drug supersaturation achieved by nano-sizing and amorphization of poorly water-soluble drug. Eur. J. Pharm. Sci., 77, 79-89 (2015). https://doi.org/10.1016/j.ejps.2015.05.027
K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. In situ molecular elucidation of drug supersaturation achieved by nano-sizing and amorphization of poorly water-soluble drug. Eur. J. Pharm. Sci., 77, 79-89 (2015). https://doi.org/10.1016/j.ejps.2015.05.027
N. Otsuka, K. Ueda, N. Ohyagi, K. Shimizu, K. Katakawa, T. Kumamoto, K. Higashi, K. Yamamoto, K. Moribe. An insight into different stabilization mechanisms of phenytoin derivatives supersaturation by HPMC and PVP. J. Pharm. Sci., 104 (8), 2574-2582 (2015). https://doi.org/10.1002/jps.24527
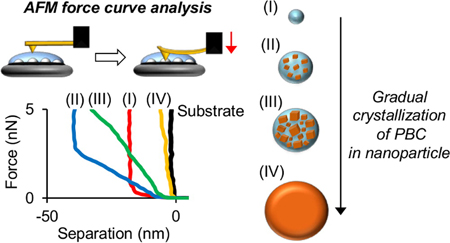 K. Egami, K. Higashi, K. Yamamoto, K. Moribe. Crystallization of probucol in nanoparticles revealed by AFM analysis in aqueous solution. Mol. Pharm., 12 (8), 2972-2980 (2015). https://doi.org/10.1021/acs.molpharmaceut.5b00236
K. Egami, K. Higashi, K. Yamamoto, K. Moribe. Crystallization of probucol in nanoparticles revealed by AFM analysis in aqueous solution. Mol. Pharm., 12 (8), 2972-2980 (2015). https://doi.org/10.1021/acs.molpharmaceut.5b00236
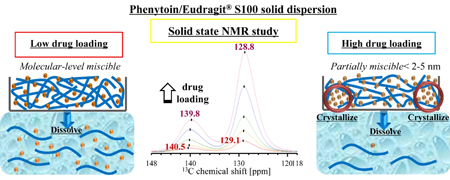 K. Higashi, H. Hayashi K. Yamamoto, K. Moribe. The effect of drug and Eudragit® S 100 miscibility in solid dispersions on the drug and polymer dissolution rate. Int. J. Pharm., 494 (1), 9-16 (2015). https://doi.org/10.1016/j.ijpharm.2015.08.007
K. Higashi, H. Hayashi K. Yamamoto, K. Moribe. The effect of drug and Eudragit® S 100 miscibility in solid dispersions on the drug and polymer dissolution rate. Int. J. Pharm., 494 (1), 9-16 (2015). https://doi.org/10.1016/j.ijpharm.2015.08.007
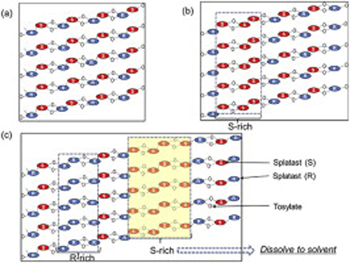 K. Nagai, T. Ushio, H. Miura, K. Moribe, K.Yamamoto, Effect of enantiotropic uniformity of polymorphic crystals on the chemical stability of suplatast tosilate. J. Drug Deliv. Sci. Technol., 27, 37-45 (2015). https://doi.org/10.1016/j.jddst.2015.04.004
K. Nagai, T. Ushio, H. Miura, K. Moribe, K.Yamamoto, Effect of enantiotropic uniformity of polymorphic crystals on the chemical stability of suplatast tosilate. J. Drug Deliv. Sci. Technol., 27, 37-45 (2015). https://doi.org/10.1016/j.jddst.2015.04.004
S. Sasaoka, K. Saito, K. Higashi, W. Limwikrant, K. Moribe, S. Suzuki, K. Yamamoto. Design of one-dimensional power spectrum using two-dimensional fast Fourier transform for discrimination of paper-based kraft tapes.Forensic Sci. Int., 257, 329-336 (2015). https://doi.org/10.1016/j.forsciint.2015.09.016
2014
M. Osawa, K. Higashi, J. Yamashita, K. Moribe, K. Yamamoto. Characterization of sulindac solid dispersion with hydroxypropyl cellulose prepared by hot melt extrusion. J. Pharm. Sci. Technol. Jpn., 74 (2), 160-169 (2014). https://doi.org/10.14843/jpstj.74.160
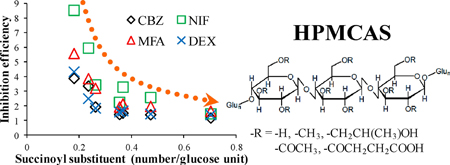 K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. The effect of HPMCAS functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int. J. Pharm., 464 (1), 205-213 (2014). https://doi.org/10.1016/j.ijpharm.2014.01.005
K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. The effect of HPMCAS functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int. J. Pharm., 464 (1), 205-213 (2014). https://doi.org/10.1016/j.ijpharm.2014.01.005
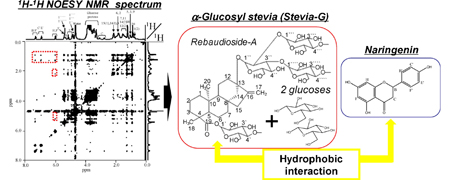 J. Zhang, K. Higashi, K. Ueda, K. Kadota, Y. Tozuka, W. Limwikrant, K. Yamamoto, K. Moribe. Drug solubilization mechanism of α-glucosyl stevia by NMR spectroscopy. Int. J. Pharm., 465 (1), 255-261 (2014). https://doi.org/10.1016/j.ijpharm.2014.01.035
J. Zhang, K. Higashi, K. Ueda, K. Kadota, Y. Tozuka, W. Limwikrant, K. Yamamoto, K. Moribe. Drug solubilization mechanism of α-glucosyl stevia by NMR spectroscopy. Int. J. Pharm., 465 (1), 255-261 (2014). https://doi.org/10.1016/j.ijpharm.2014.01.035
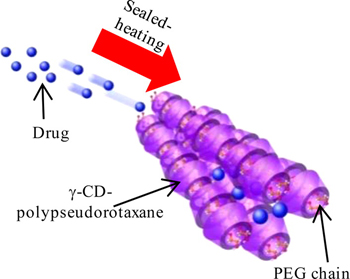 K. Higashi, H. Waraya, L. K. Lin, S. Namiki, M. Ogawa, W. Limwikrant, K. Yamamoto, K. Moribe. Application of intermolecular spaces between polyethylene glycol/γ-cyclodextrin-polypseudorotaxanes as a host for various guest drugs. Cryst. Growth Des., 14 (6), 2773-2781 (2014). https://doi.org/10.1021/cg401934v
K. Higashi, H. Waraya, L. K. Lin, S. Namiki, M. Ogawa, W. Limwikrant, K. Yamamoto, K. Moribe. Application of intermolecular spaces between polyethylene glycol/γ-cyclodextrin-polypseudorotaxanes as a host for various guest drugs. Cryst. Growth Des., 14 (6), 2773-2781 (2014). https://doi.org/10.1021/cg401934v
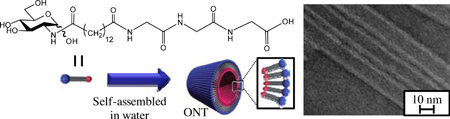 K. Moribe, T. Makishima, K. Higashi, N. Liu, W. Limwikrant, W. Ding, M. Masuda, T. Shimizu, K. Yamamoto. Encapsulation of poorly water-soluble drugs into organic nanotubes for improving drug dissolution. Int. J. Pharm., 469 (1), 190-196 (2014). https://doi.org/10.1016/j.ijpharm.2014.04.005
K. Moribe, T. Makishima, K. Higashi, N. Liu, W. Limwikrant, W. Ding, M. Masuda, T. Shimizu, K. Yamamoto. Encapsulation of poorly water-soluble drugs into organic nanotubes for improving drug dissolution. Int. J. Pharm., 469 (1), 190-196 (2014). https://doi.org/10.1016/j.ijpharm.2014.04.005
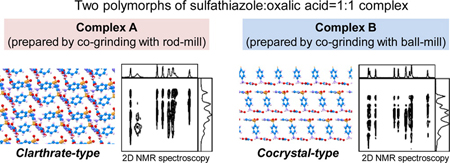 R. Koike, K. Higashi, N. Liu, W. Limwikrant, K. Yamamoto, K. Moribe. Structural determination of a novel polymorph of sulfathiazole-oxalic acid complex in powder form by solid-state NMR spectroscopy on the basis of crystallographic structure of another polymorph. Cryst. Growth Des., 14 (9), 4510-4518 (2014). https://doi.org/10.1021/cg5005903
R. Koike, K. Higashi, N. Liu, W. Limwikrant, K. Yamamoto, K. Moribe. Structural determination of a novel polymorph of sulfathiazole-oxalic acid complex in powder form by solid-state NMR spectroscopy on the basis of crystallographic structure of another polymorph. Cryst. Growth Des., 14 (9), 4510-4518 (2014). https://doi.org/10.1021/cg5005903
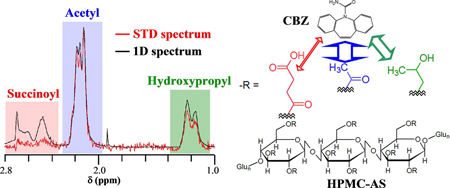
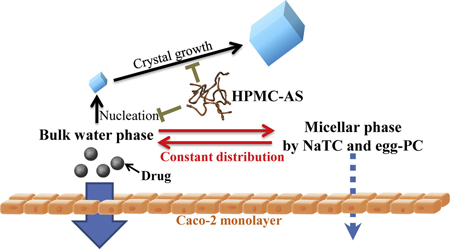 K. Ueda, K. Higashi, M. Kataoka, S. Yamashita, K. Yamamoto, K. Moribe. Inhibition mechanism of hydroxypropyl methylcellulose acetate succinate on drug crystallization in gastrointestinal fluid and drug permeability from a supersaturated solution. Eur. J. Pharm. Sci., 62, 293-300 (2014). https://doi.org/10.1016/j.ejps.2014.06.007
K. Ueda, K. Higashi, M. Kataoka, S. Yamashita, K. Yamamoto, K. Moribe. Inhibition mechanism of hydroxypropyl methylcellulose acetate succinate on drug crystallization in gastrointestinal fluid and drug permeability from a supersaturated solution. Eur. J. Pharm. Sci., 62, 293-300 (2014). https://doi.org/10.1016/j.ejps.2014.06.007
R. Chiba, Y. Kuroiwa, K. Higashi, K. Yamamoto, K. Moribe. Characterization of as-synthesized mesoporous silica using NMR and solid-state fluorescence spectroscopy. J. Drug Deliv. Sci. Technol., 24 (6), 673-677 (2014). https://doi.org/10.1016/S1773-2247(14)50135-9
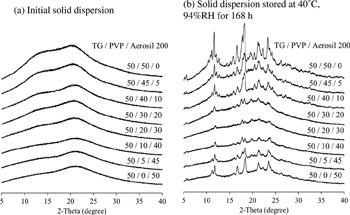 T. Nakahashi, T. Matsumoto, N. Wakiyama, K. Moribe, K. Yamamoto. The role of light anhydrous silicic acid on physical stability of troglitazone solid dispersion under humidified conditions. Adv. Powder Tech., 25 (2), 716-721 (2014). https://doi.org/10.1016/j.apt.2013.10.020
T. Nakahashi, T. Matsumoto, N. Wakiyama, K. Moribe, K. Yamamoto. The role of light anhydrous silicic acid on physical stability of troglitazone solid dispersion under humidified conditions. Adv. Powder Tech., 25 (2), 716-721 (2014). https://doi.org/10.1016/j.apt.2013.10.020
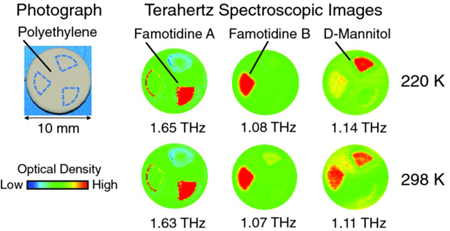 K. Ajito, Jae-Young Kima, Y. Ueno, Ho-Jin Songa, K. Ueda, W. Limwikrant, K. Yamamoto, K. Moribe. Nondestructive multicomponent terahertz chemical imaging of medicine in tablets. J. Electrochem. Soc., 161 (9), B171-B175 (2014). http://doi.org/10.1149/2.0201409jes
K. Ajito, Jae-Young Kima, Y. Ueno, Ho-Jin Songa, K. Ueda, W. Limwikrant, K. Yamamoto, K. Moribe. Nondestructive multicomponent terahertz chemical imaging of medicine in tablets. J. Electrochem. Soc., 161 (9), B171-B175 (2014). http://doi.org/10.1149/2.0201409jes
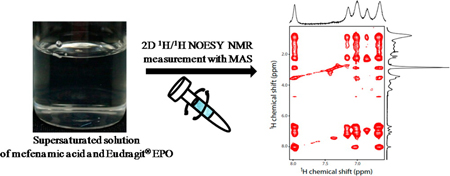 K. Higashi, K. Yamamoto, M. K. Pandey, K. H. Mroue, K. Moribe, K. Yamamoto, A. Ramamoorthy. Insights into atomic-level interaction between mefenamic acid and Eudragit EPO in a supersaturated solution by high-resolution magic-angle spinning NMR Spectroscopy. Mol. Pharm., 11 (1), 351-357 (2014). https://doi.org/10.1021/mp4005723
K. Higashi, K. Yamamoto, M. K. Pandey, K. H. Mroue, K. Moribe, K. Yamamoto, A. Ramamoorthy. Insights into atomic-level interaction between mefenamic acid and Eudragit EPO in a supersaturated solution by high-resolution magic-angle spinning NMR Spectroscopy. Mol. Pharm., 11 (1), 351-357 (2014). https://doi.org/10.1021/mp4005723
 K. Nagai, T. Ushio, H. Miura, T. Nakamura, K. Moribe, K. Yamamoto. Four new polymorphic forms of suplatast tosilate. Int. J. Pharm., 460 (1-2), 83-91 (2014) https://doi.org/10.1016/j.ijpharm.2013.10.049
K. Nagai, T. Ushio, H. Miura, T. Nakamura, K. Moribe, K. Yamamoto. Four new polymorphic forms of suplatast tosilate. Int. J. Pharm., 460 (1-2), 83-91 (2014) https://doi.org/10.1016/j.ijpharm.2013.10.049
2013
M. Fushimi, K. Moribe, K. Higashi, K. Yamamoto. Intermolecular interaction between low-substituted hydroxypropylcellulose and water-soluble drugs in granules prepared by wet granulation. J. Pharm. Sci. Technol. Jpn. , 73(1), 72-81 (2013). https://doi.org/10.14843/jpstj.73.72
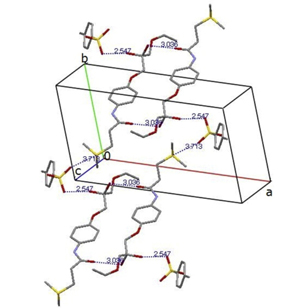
 M. Umino, K. Higashi, H. Masu, W. Limwikrant, K. Yamamoto, K. Moribe. Characterization of cromolyn sodium hydrates and its formulation by 23Na-multiquantum and magic-angle spinning nuclear magnetic resonance spectroscopy. J. Pharm. Sci., 102(8), 2738-2747 (2013). https://doi.org/10.1002/jps.23655
M. Umino, K. Higashi, H. Masu, W. Limwikrant, K. Yamamoto, K. Moribe. Characterization of cromolyn sodium hydrates and its formulation by 23Na-multiquantum and magic-angle spinning nuclear magnetic resonance spectroscopy. J. Pharm. Sci., 102(8), 2738-2747 (2013). https://doi.org/10.1002/jps.23655
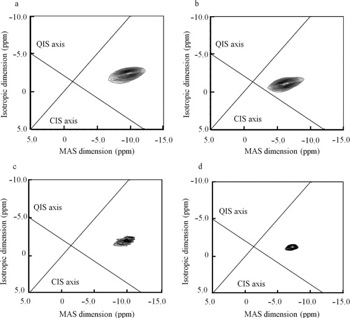
 K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. Inhibitory effect of hydroxypropyl methylcellulose acetate succinate on drug recrystallization from a supersaturated solution assessed using nuclear magnetic resonance measurements. Mol. Pharm., 10(10), 3801-3811 (2013). https://doi.org/10.1021/mp400278j
K. Ueda, K. Higashi, K. Yamamoto, K. Moribe. Inhibitory effect of hydroxypropyl methylcellulose acetate succinate on drug recrystallization from a supersaturated solution assessed using nuclear magnetic resonance measurements. Mol. Pharm., 10(10), 3801-3811 (2013). https://doi.org/10.1021/mp400278j
K. Moribe, K. Ueda, W. Limwikrant, K. Higashi, K. Yamamoto,. Nano-sized crystalline drug production by milling technology. Curr. Pharm. Des., 19(35), 6246-6258 (2013). https://doi.org/10.2174/1381612811319350003
F. Uejo, W. Limwikrant, K. Moribe, K. Yamamoto. Dissolution improvement of fenofibrate by melting inclusion in mesoporous silica. Asian J. Pharm. Sci., 8(6), 329-335 (2013). https://doi.org/10.1016/j.ajps.2013.11.001
