Summary of Research Projects
Our basic research objective is chemical, synthetic, and medicinal study on biologically active natural organic molecules produced by botanical medicinal resources. Following are three main ways of approach that we employ.
1) Survey of natural molecules of high potential use in medicinal resources (isolation, structure elucidation by means of spectroscopy and chemical reaction of natural molecules, etc.)
We investigate "seed molecules" in medicinal plants distributed
in Japan, East Asia, and Southeast Asia. A major part of our recent research
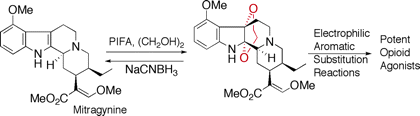
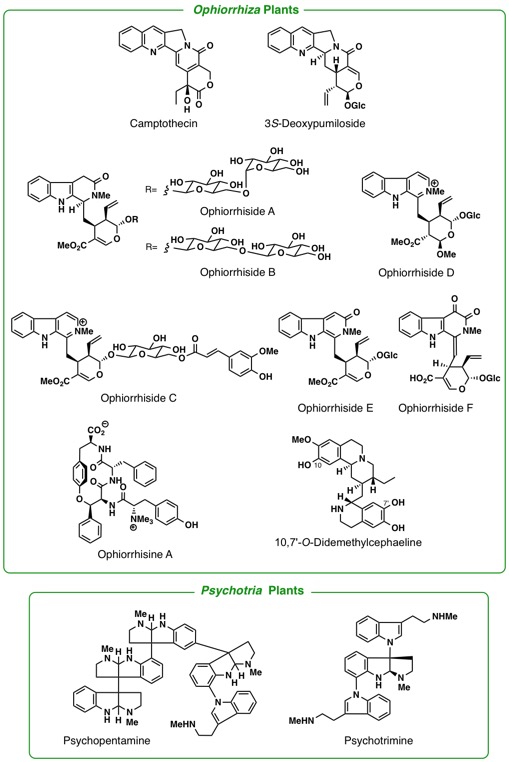
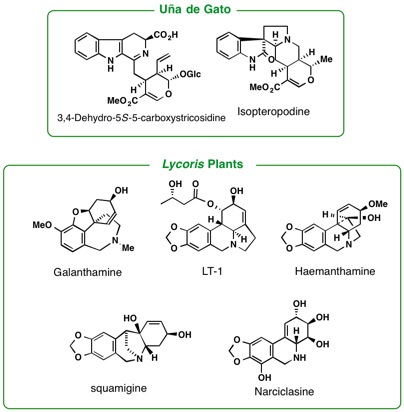
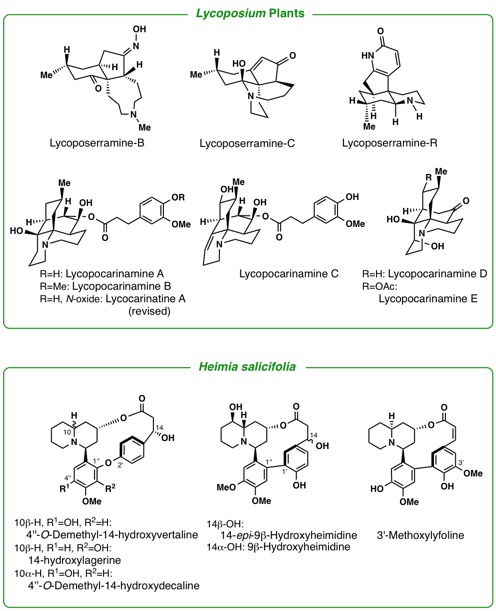
orients to Rubiaceae, including the genera, Mitragyna, Ophiorrhiza, and Adina. The leaves of a tropical plant, Mitragyna speciosa Korth., have been traditionally used as a substitute for opium. By phytochemical
studies on the constituents of the plant growing in Thailand as well as
in Malaysia, we have isolated 7-hydroxymitragynine, a minor constituent
of this plant, which was found to exhibit potent opioid agonistic properties
in vitro and in vivo experiments. Some synthetic derivatives of 7-hydroxymitragynine
were found to possess potent and useful antinociceptive activity in mice.
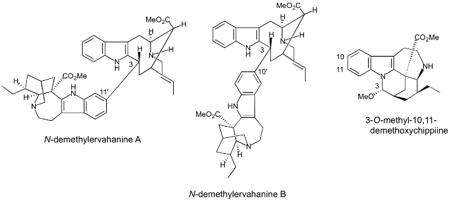
A loganiaceous plant Gelsemium elegans is an extremely poisonous plant found in southeast-Asia. Chemical
investigation of the Gelsemium plants in detail led us to isolate more than seventy kinds of indole-related
alkaloids having complex and various types of structures. Among them, some
alkaloids were proved to exhibit highly potent antitumor activity. To investigate
the seed molecules effective to Alzheimer’s disease we study the alkaloidal
constituents in the plants belonging to Lycopodiaceae, Calycanthaceae,
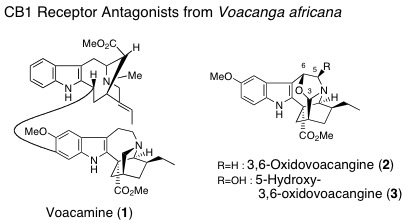
and Amaryllidaceae. Iboga-type alkaloids in Voacanga africana were found to have TRP channel antagonistic activity. Investigations of
pyrrolizidine alkaloids in asteraceous and boraginaceous plants, some of
which are known as naturally occurring toxins, are under way. Other plant
genera of our close concern are Kopsia, Voacanga, and Ervatamia (Apocynaceae), Pandanus (Pandanaceae), Heimia(Lythraceae), and Lycoris (Liliaceae).
To study constituents of foreign plant species we have close collaborative
relationships to phytochemists of the universities or institutes of the
plant producing countries.
Recent Literatures
M. Kitajima, Y. Yamaguchi, T. Yanagisawa, N. Kogure, J. Ogata, R. Kikura-Hanajiri,
and H. Takayama:
Biphenyl quinolizidine lactone alkaloids from "sinicuichi" (Heimia salicifolia).
Tetrahedron,75(27), 3733-3739 (2019).
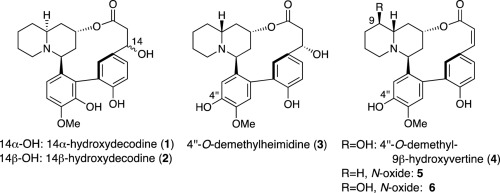
M. Kitajima, K. Okabe, M. Yoshida, R. Nakabayashi, K, Saito, N. Kogure,
and H. Takayama:
New Otonecine-type Pyrrolizidine Alkaloid from Petasites japonicus.
J. Nat. Med.,73(3), 602-607 (2019).
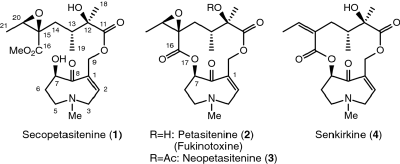
M. Kitajima, S. Nakano, N. Kogure, S. Subhadhirasakul, and H. Takayama:
New Indole Alkaloids from Ervatamia cumingiana.
Heterocycles,99(1), 213-221 (2019).
M. Kitajima, T. Yanagisawa, M. Tsukahara, Y. Yamaguchi, N. Kogure, R. Kikura-Hanajiri,
Y. Goda, O. Iida, Y. Sugimura, N. Kawahara, and H. Takayama:
Biphenyl Ether and Biphenyl Quinolizidine Lactone Alkaloids from Heimia salicifolia.
Tetrahedron,74(4), 441-452 (2018).
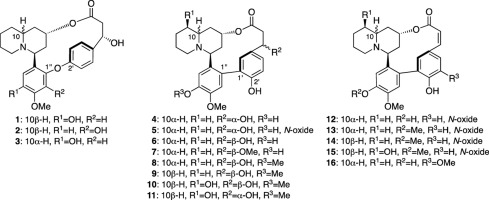
T. Onozawa, M. Kitajima, N. Kogure, N. Peerakam, D. Santiarworn, and H.
Takayama:
A Cyclopeptide and a Tetrahydroisoquinoline Alkaloid from Ophiorrhiza nutans.
J. Nat. Prod.,80(7), 2156-2160 (2017).
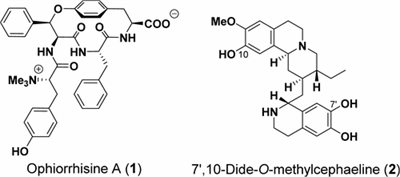
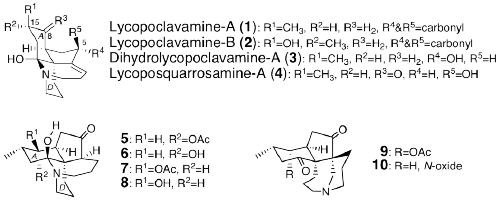
N. Kogure, M. Maruyama, S. Wongseripipatana, M. Kitajima, and H. Takayama:
New Lycopodine-type Alkaloids from Lycopodium carinatum.
Chem. Pharm. Bull.,64(7), 793-799 (2016).
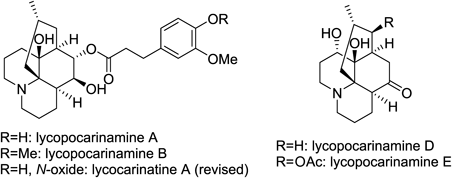
M. Kitajima, M. Nakazawa, Y. Wu, N. Kogure, R. Zhang, and H. Takayama:
Kopsiyunnanines L and M, Strychnos-related Monoterpenoid Indole Alkaloids
from Yunnan Kopsia arborea.
Tetrahedron,72(42), 6692-6696 (2016).
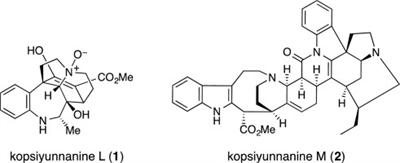
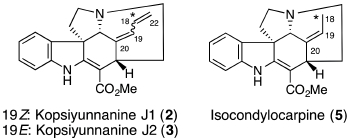
M. Kitajima, T. Koyama, Y. Wu, N. Kogure, R. Zhang, and H. Takayama:
Kopsiyunnanines J1 and J2, New Strychnos-type Homo-Monoterpenoid Indole
Alkaloids from Kopsia arborea.
Nat. Prod. Commun.,10(1), 49-51 (2015).
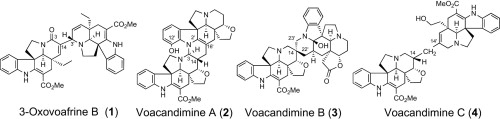
M. Kitajima, M. Iwai, N. Kogure, R. Kikura-Hanajiri, Y. Goda, and H. Takayama
:
Aspidosperma-aspidosperma-type Bisindole Alkaloids from Voacanga africana.
Tetrahedron,69(2) , 796-801 (2013).
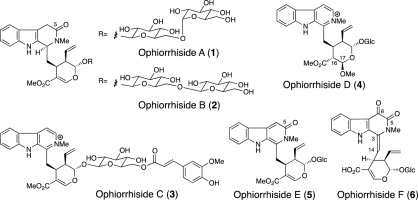
M. Kitajima, S. Ohara, N. Kogure, D. Santiarworn, and H. Takayama:
β-Carboline-type indole alkaloid glycosides from Ophiorrhiza trichocarpon.
Tetrahedron,69(45) , 9451-9456 (2013).
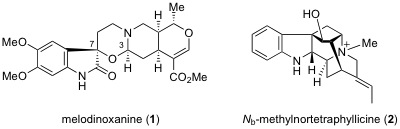
M. Kitajima, S. Ohara, N. Kogure, Y. Wu, R. Zhang, and H. Takayama :
New Indole Alkaloids from Melodinus henryi.
Heterocycles,85(8), 1949-1959 (2012).
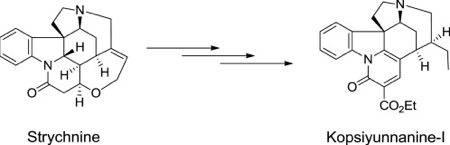
N. Kogure, Y. Suzuki, Y. Wu, M. Kitajima, R. Zhang, and H. Takayama :
Chemical Conversion of Strychnine into Kopsiyunnanine-I, a New Hexacyclic
Indole Alkaloid from Yunnan Kopsia arborea.
Tetrahedron Lett.,53(48), 6523-6526 (2012).
K. Katakawa, H. Mito, N. Kogure, M. Kitajima, S. Wongseripipatana, M. Arisawa,
and H. Takayama :
Ten New Fawcettimine-related Alkaloids from Three Species of Lycopodium.
Tetrahedron,67(35) 6561-6567 (2011).
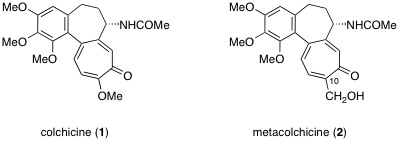
M. Kitajima, A. Tanaka, N. Kogure, and H. Takayama :
Metacolchicine, A New Colchicinoid from Sandersonia aurantiaca.
Heterocycles,83,1903-1907 (2011).
Y. Yamada, M. Kitajima, S. Wongseripipatana, N. Kogure, and H. Takayama
:
Seven New Monoterpenoid Indole Alkaloids from Gelsemium elegans.
Chem. Asian J.,6(1), 166-173 (2011).
Y. Wu, M. Kitajima, N. Kogure, Y. Wang, R. Zhang, and H. Takayama :
Two New Aspidosperma Indole Alkaloids from Yunnan Kopsia arborea.
Chem. Pharm. Bull.,58(7), 961-963 (2010).
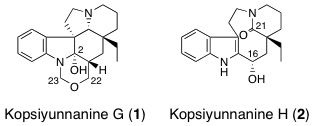
M. A. Tan, M. Kitajima, N. Kogure, M. G. Nonato, and H. Takayama :
New Pyrrolidine Alkaloids from the Roots of Pandanus amaryllifolius.
Tetrahedron Lett.,51(31), 4143-4146 (2010).
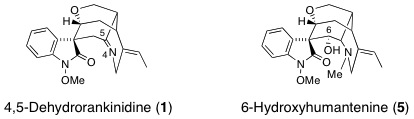
M. Kitajima, H. Kobayashi, N. Kogure, and H. Takayama :
New Oxindole and Indole Alkaloids from Gelsemium rankinii.
Tetrahedron,66(32), 5987-5992 (2010).
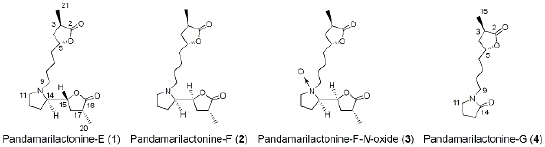
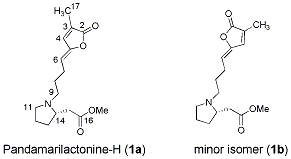
M. A. Tan, M. Kitajima, N. Kogure, M. G. Nonato, and H. Takayama :
Isolation of Pandamarilactonine-H from the Roots of Pandanus amaryllifolius and Synthesis of epi-Pandamarilactonine-H.
J. Nat. Prod.,73(8), 1453-1455 (2010).
Hasnah M. Sirat, Deny Susanti, Farediah Ahmad, H. Takayama, and M. Kitajima
:
Amide, Triterpene and Flavonoids from the Leaves of Malastoma malabathricum
L.
J. Nat. Med.,64(4), 492-495 (2010).
Y. Yamada, M. Kitajima, N. Kogure, S. Wongseripipatana, and H. Takayama
:
Spectroscopic Analyses and Chemical Transformation for
Structure Elucidation of Two Novel Indole Alkaloids from
Gelsemium elegans.
Tetrahedron Lett.,50(26), 3341-3344 (2009).
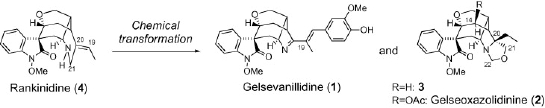
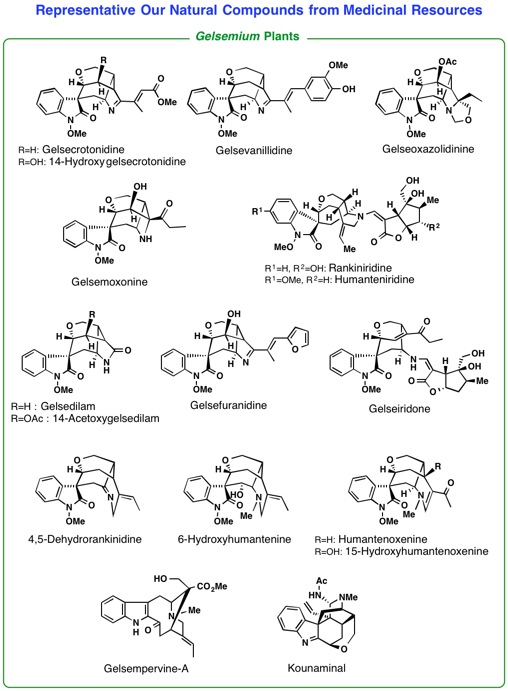
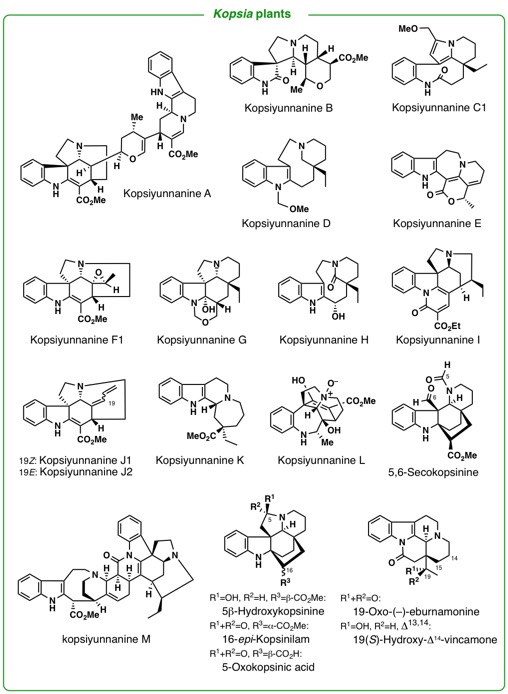
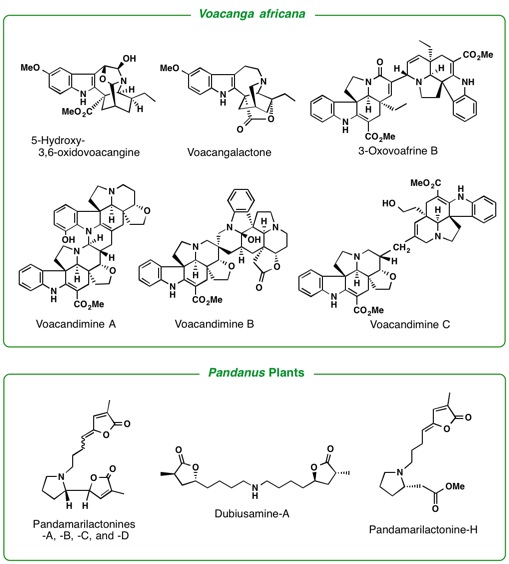
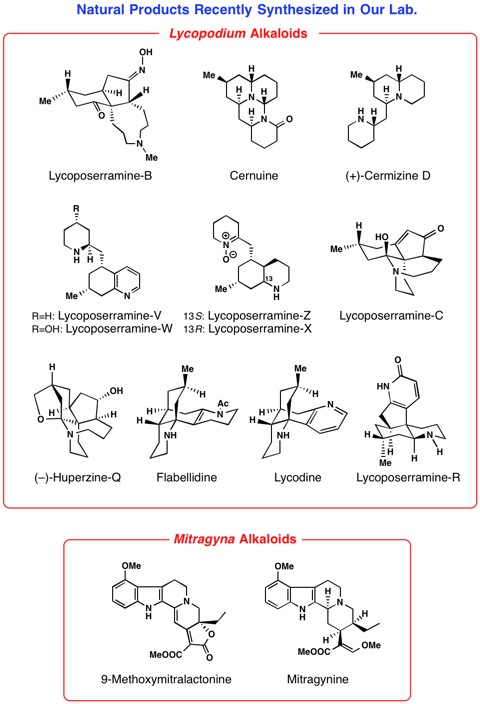
2) Studies on synthesis of biologically active natural molecules isolated by our hands (asymmetric total synthesis, and chemical conversion of biologically active natural products).
We try to develop efficient synthetic routes to targeted organic molecules
that have been isolated in our laboratory and have biological functions.
We aim to develop an enantioselective route whenever possible and we try
to design the sequence of chemical reactions by taking the idea from biosynthetic
pathways.
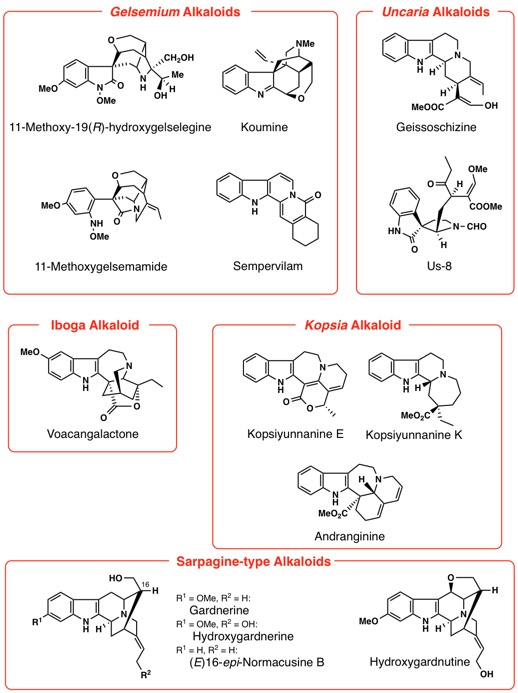
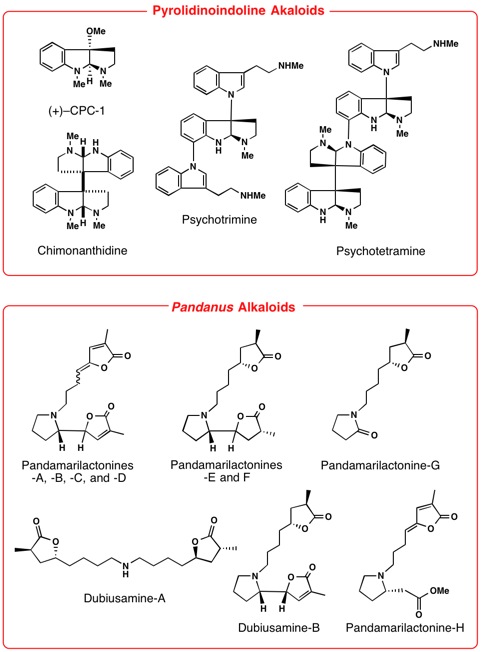
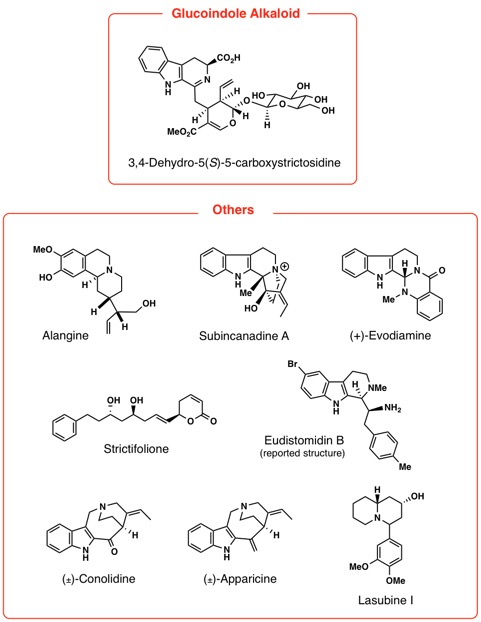
Our recent research works are syntheses of Lycopodium, Kopsia, Voacanga, Mitragyna, Gelsemium, Heimia, Pandanus, Strychnos,
Aspidosperma, Chimonanthus, and Lycoris alkaloids. Thus, we have achieved first asymmetric total synthesis of
some of the structurally complex alkaloids.
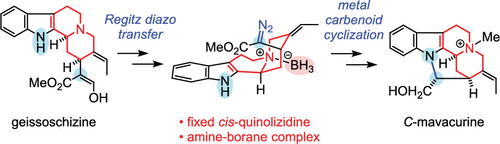
K. Sato, N. Kogure, M. Kitajima, and H. Takayama:
Total Syntheses of Pleiocarpamine, Normavacurine, and C-Mavacurine.
Org. Lett.,21(9), 3342-3345 (2019).
H. Kaneko, S. Takahashi, N. Kogure, M. Kitajima, and H. Takayama:
Asymmetric Total Synthesis of Fawcettimine-Type Lycopodium Alkaloid, Lycopoclavamine-A.
J. Org. Chem.,84(9), 5645-5654 (2019).
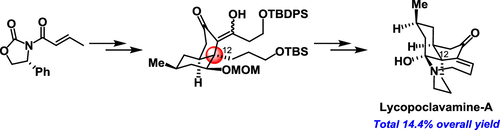
K. Wada, N. Kogure, M. Kitajima, and H. Takayama:
Concise Asymmetric Total Synthesis of Lycopodine and Flabelliformine via
Cascade Cyclization Reaction.
Tetrahedron Lett.,60(2), 187-190 (2019).
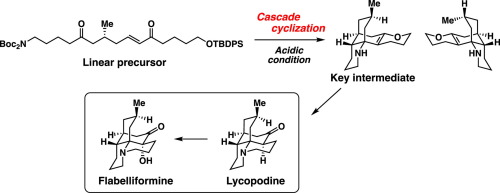
K. Sato, N. Takanashi, N. Kogure, M. Kitajima, and H. Takayama:
Formal Total Synthesis of (±)-Strictamine.
Heterocycles,97(1), 365-382 (2018).
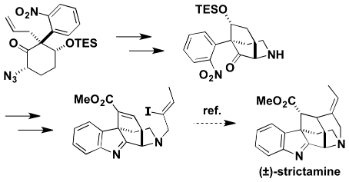
T. Onozawa, M. Kitajima, N. Kogure, and H. Takayama:
Asymmetric Total Synthesis and Evaluation of Antitumor Activity of Ophiorrhisine
A and Its Derivatives.
J. Org. Chem.,83(24), 15312-15322 (2018).
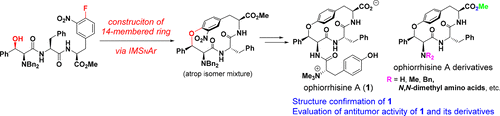
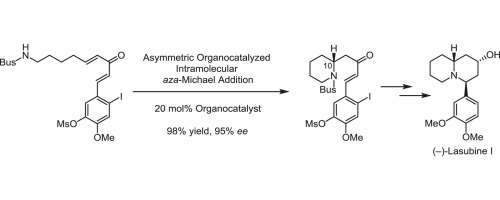
T. Hirama, T. Umemura, N. Kogure, M. Kitajima, and H. Takayama:
Synthetic Study of Biphenylquinolizidine Alkaloids I. Asymmetric Total
Synthesis of Lasubine I Featuring Organocatalyzed Asymmetric Intramolecular
aza-Michael Addition.
Tetrahedron Lett.,58(3), 223-226 (2017).
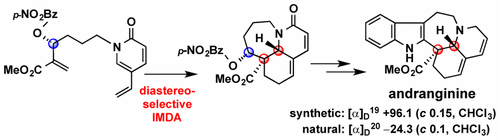
S. Tooriyama, Y. Mimori, Y. Wu, N. Kogure, M. Kitajima, and H. Takayama:
Asymmetric Total Synthesis of Pentacyclic Indole Alkaloid Andranginine
and Absolute Configuration of Natural Product Isolated from Kopsia arborea.
Org. Lett.,19(10), 2722-2725 (2017).
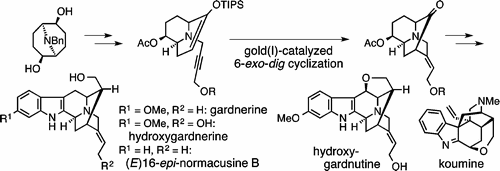
M. Kitajima, K. Watanabe, H. Maeda, N. Kogure, and H. Takayama:
Asymmetric Total Synthesis of Sarpagine-Related Indole Alkaloids Hydroxygardnerine,
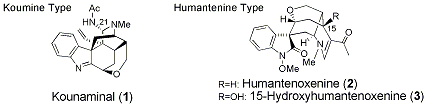
Hydroxygardnutine, Gardnerine, (E)-16-epi-Normacusine B, and Koumine.
Org. Lett.,18(8), 1912-1915 (2016).
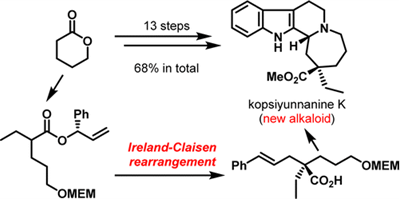
R. Tokuda, Y. Okamoto, T. Koyama, N. Kogure, M. Kitajima, and H. Takayama:
Asymmetric Total Synthesis of Kopsiyunnanine K, a Monoterpenoid Indole
Alkaloid with a Rearranged Skeleton.
Org. Lett.,18(14), 3490-3493 (2016).
N. Takanashi, K. Suzuki, M. Kitajima, and H. Takayama:
Total Synthesis of Conolidine and Apparicine.
Tetrahedron Lett.,57(3), 375-378 (2016).
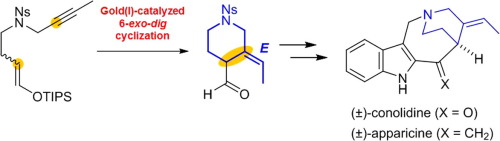
H. Ishida, S. Kimura, N. Kogure, M. Kitajima, and H. Takayama:
Total Synthesis of (±)-Lycoposerramine-R, a Novel Skeletal Type of Lycopodium Alkaloid.
Tetrahedron,71(1), 51-56 (2015).
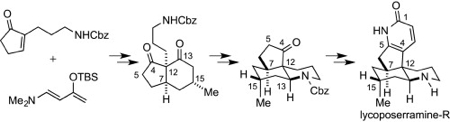
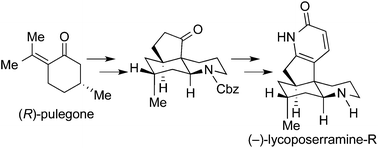
H. Ishida, S. Kimura, N. Kogure, M. Kitajima, and H. Takayama:
The First Asymmetric Total Synthesis of Lycoposerramine-R.
Org. Biomol. Chem.,13(28), 7762-7771 (2015).
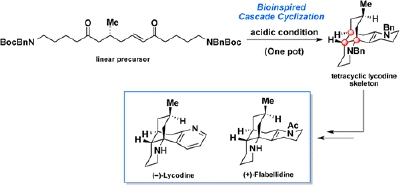
M. Azuma, T. Yoshikawa, N. Kogure, M. Kitajima, and H. Takayama:
Biogenetically Inspired Total Syntheses of Lycopodium Alkaloids, (+)-Flabellidine and (–)-Lycodine.
J. Am. Chem. Soc.,136(33), 11618-11621 (2014).
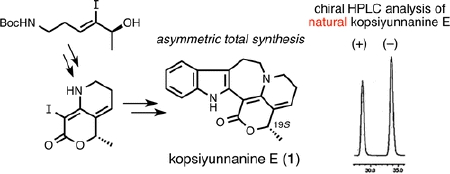
M. Kitajima, Y. Murakami, N. Takahashi, Y. Wu, N. Kogure, R. Zhang, and
H. Takayama:
Asymmetric Total Synthesis of Novel Pentacyclic Indole Alkaloid, Kopsiyunnanine
E, Isolated from Kopsia arborea.
Org. Lett.,16(19), 5000-5003 (2014).
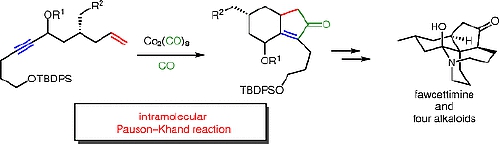
A. Nakayama, M. Kitajima, and H. Takayama :
Syntheses of Fawcettimine-type Lycopodium Alkaloids Utilizing Pauson-Khand Reaction.
Synlett,23(14), 2014-2024 (2012) Invited Account.
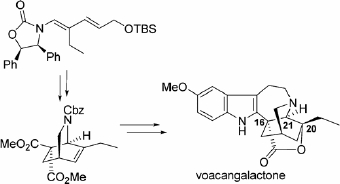
M. Harada, K. Asaba, M. Iwai, N. Kogure, M. Kitajima and H. Takayama :
Asymmetric Total Synthesis of an Iboga-Type Indole Alkaloid, Voacangalactone,
Newly Isolated from Voacanga africana.
Org. Lett.,14(22), 5800-5803 (2012).
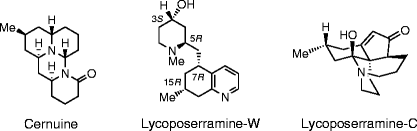
M. Kitajima and H. Takayama :
Lycopodium Alkaloids: Isolation and Asymmetric Synthesis.
Topics in Current Chemistry, “Alkaloid Synthesis”, ed. by H.-J. Knölker, Springer, 309, 1-32 (2012).
A. Nakayama, N. Kogure, M. Kitajima, and H. Takayama :
Asymmetric Total Synthesis of a Pentacyclic Lycopodium Alkaloid, Huperzine-Q.
Angew. Chem., Int. Ed.,50(35). 8025-8028 (2011).
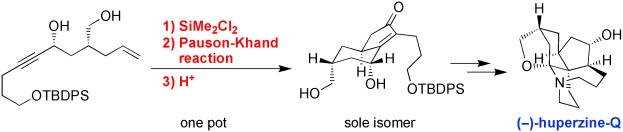
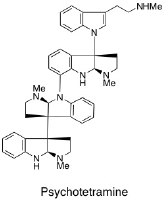
K. Foo, T. Newhouse, I. Mori, H. Takayama, and P. S. Baran :
Total Synthesis Guided Structure Elucidation of (+)-Psychotetramine.
Angew. Chem., Int. Ed.,50(12), 2716-2719 (2011).
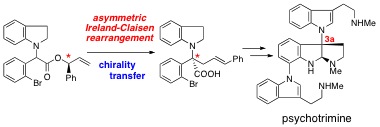
N. Takahashi, T. Ito, Y. Matsuda, N. Kogure, M. Kitajima, and H. Takayama
:
Determination of Absolute Configuration of Trimeric Indole Alkaloid, Psychotrimine,
by First Asymmetric Total Synthesis.
Chem. Commun.,46(14), 2501-2503 (2010).
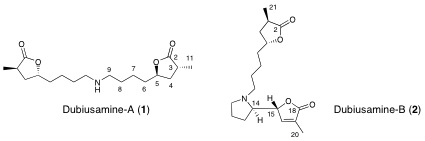
M. A. Tan, M. Kitajima, N. Kogure, M. G. Nonato, and H. Takayama :
Isolation and Total Syntheses of Two New Alkaloids, Dubiusamines-A and-B,
from Pandanus dubius.
Tetrahedron,66(18), 3353-3359 (2010).
A. Nakayama, N. Kogure, M. Kitajima, and H. Takayama :
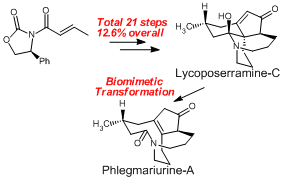
First Asymmetric Total Syntheses of Fawcettimine-Type Lycopodium Alkaloids, Lycoposerramine-C and Phlegmariurine-A.
Org. Lett.,11(23), 5554-5557 (2009).
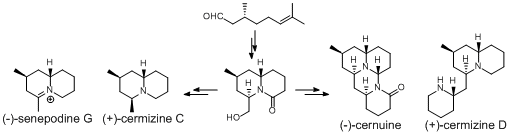
Y. Nishikawa, M. Kitajima, N. Kogure, and H. Takayama :
A Divergent Approach for the Total Syntheses of Cernuane-type and Quinolizidine-type
Lycopodium Alkaloids.
Tetrahedron,65(8), 1608-1617 (2009).
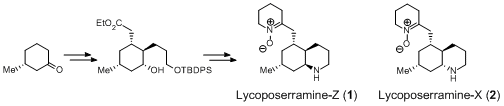
T. Tanaka, N. Kogure, M. Kitajima, and H. Takayama :
Asymmetric Total Syntheses of Cyclic-Nitrone-Containing Phlegmarine-Type
Lycopodium Alkaloids, Lycoposerramines-X and -Z.
J. Org. Chem.,74(22), 8675-8680 (2009).
3)Medicinal chemistry research by using our libraries of natural and synthetic compounds, and study on the action mechanisms of the biologically active compounds in molecular level.
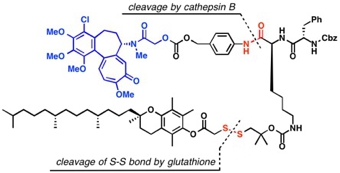
Our collaborative researchers in the same faculty or outside of the university evaluate our natural or synthetic molecules in respect to the pharmacological activities, and the results obtained there help us to design new molecular structures for the study of medicinal chemistry. Some of our recent researches concerned are the opioid agonistic activity of Mitragyna alkaloids, antitumor activity of Gelsemium alkaloids, Kopsia alkaloids, and colchicinoids, alkaloids possessing AChE inhibitory activity found in Lycopodiaceae, Calycanthaceae, and Amaryllidaceae, and Iboga-type alkaloids possessing TRP channel or cannabinoid CB1 antagonistic activities.
M. Kitajima, A. Morita, S. Endo, N. Kogure, K. Higashi, K. Moribe, and
H. Takayama:
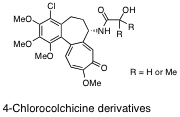
Design and Synthesis of 4-Chlorocolchicine-Derived Prodrug Capable of Forming
Nanoparticles by Self-assembly.
Heterocycles,95(1), 181-186 (2017).
K. Matsumoto, M. Narita, N. Muramatsu, T. Nakayama, K. Misawa, M. Kitajima,
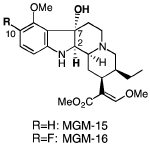
K. Tashima, T. Suzuki, H. Takayama, and S. Horie:
The Orally Active Opioid μ/δ Dual Agonist MGM-16, a Derivative of Indole
Alkaloid Mitragynine, Exhibits Potent Analgesic Effect on Neuropathic Pain
in Mice.
J. Pharmacol. Exp. Ther.,348(3), 383-392 (2014).
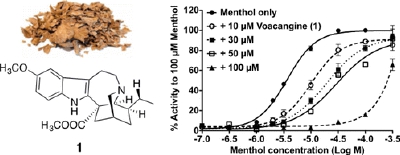
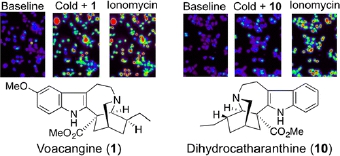
Y. Terada, S. Horie, H. Takayama, K. Uchida, M. Tominaga, and T. Watanabe:
Activation and Inhibition of Thermosensitive TRP Channels by Voacangine,
an Alkaloid Present in Voacanga Africana, an African Tree.
J. Nat. Prod.,77(2), 285-297 (2014).
H. Nishiyama, M. Ono, T. Sugimoto, T. Sasai, N. Asakawa, S. Ueno, Y. Tominaga,
T. Yaegashi, M Nagaoka, T Matsuzaki, N Kogure, M. Kitajima, and H. Takayama:
4-Chlorocolchicine Derivatives Bearing a Thiourea Side Chain at the C-7
Position as Potent Anticancer Agents.
Med. Chem. Commun.,5(4), 452-458 (2014).
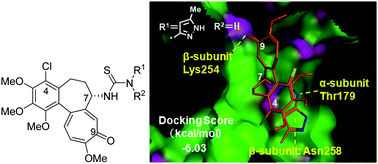
Y. Terada, M. Kitajima, F. Taguchi, H. Takayama, S. Horie, and T. Watanabe:
Identification of Indole Alkaloid Structural Units Important for Stimulus-Selective
TRPM8 Inhibition: SAR Study of Naturally Occurring Iboga Derivatives.
J. Nat. Prod.,77(8), 1831-1838 (2014).
H. Nishiyama, M. Ono, T. Sugimoto, T. Sasai, N. Asakawa, T. Matsumoto,
T. Yaegashi, M. Nagaoka, T. Matsuzaki, N. Kogure, M. Kitajima, and H. Takayama
:
Synthesis and Biological Evaluation of 4-Chlorocolchicine Derivatives as
Potent Anticancer Agents with Broad Effective Dosage Ranges.
Med. Chem. Comm.,3(12), 1500-1504 (2012).
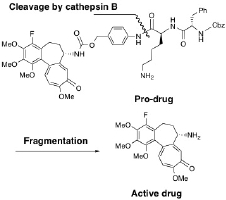
N. Yasobu, M. Kitajima, N. Kogure, Y. Shishido, T. Matsuzaki, M. Nagaoka,
and H. Takayama : Design, Synthesis, and Anti-tumor Activity of 4-Halocolchicines
and their Pro-drugs Activated by Cathepsin B.
ACS Med. Chem. Lett.,2(5), 348-352 (2011).
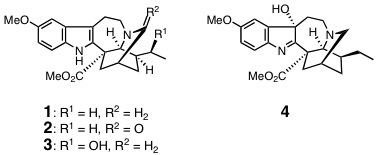
M. Kitajima, M. Iwai, R. Kikura-Hanajiri, Y. Goda, M. Iida, H. Yabushita,
and H. Takayama : Discovery of Indole Alkaloids with Cannabinoid CB1 Receptor
Antagonistic Activity.
Bioorg. Med. Chem. Lett.,21, 1962-1964 (2011).
M. Lo, K. Matsumoto, M. Iwai, K. Tashima, M. Kitajima, S. Horie, and H.
Takayama :
Inhibitory Effect of Iboga-type Indole Alkaloids on Capsaicin-induced Contraction
in Isolated Mouse Rectum.
J. Nat. Med.,65(1), 157-165 (2011).
H. Takayama, Y. Yaegashi, M. Kitajima, X. Hanb, K. Nishimura, S. Okuyama
and K. Igarashi :
Design, synthesis, and biological evaluation of tricyclic heterocycle-tetraamine
conjugates as potent NMDA channel blockers.
Bioorg. Med. Chem. Lett.,17(17), 4729-4732 (2007).
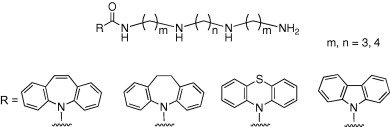
H. Takayama, K. Misawa, N. Okada, H. Ishikawa, M. Kitajima, Y. Hatori,
T. Murayama,
S. Wongseripipatana, K. Tashima, K. Matsumoto, and S. Horie :
New Procedure to Mask the 2,3-π Bond of the Indole Nucleus
and Its Application to the Preparation of Potent Opioid
Recepter Agonists with a Corynanthe Skeleton.
Org. Lett.,8(25), 5705-5708 (2006).